Home /
Expert Answers /
Chemical Engineering /
you-decided-to-use-tlc-to-determine-the-polarity-of-the-unknown-sample-with-the-molecular-formula-c-pa189
(Solved): You decided to use TLC to determine the polarity of the unknown sample with the molecular formula C ...
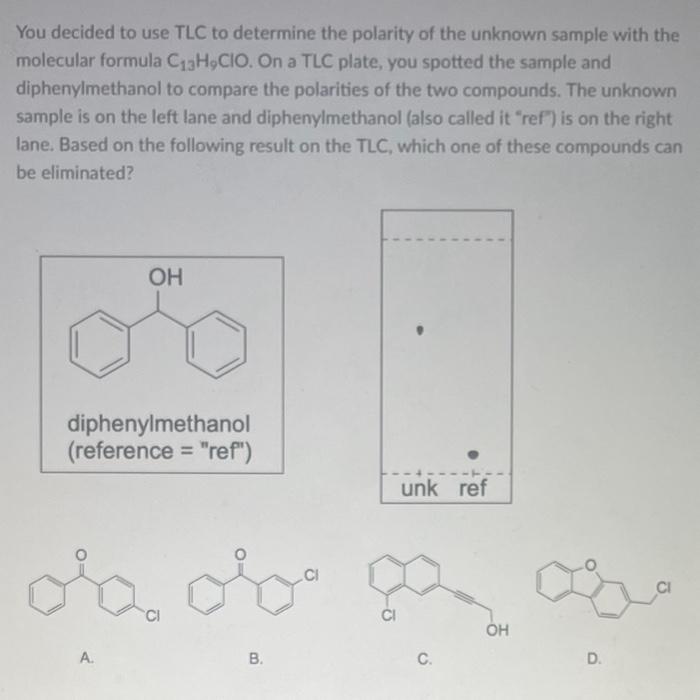
You decided to use TLC to determine the polarity of the unknown sample with the molecular formula . On a TLC plate, you spotted the sample and diphenylmethanol to compare the polarities of the two compounds. The unknown sample is on the left lane and diphenylmethanol (also called it "ref") is on the right lane. Based on the following result on the TLC, which one of these compounds can be eliminated? diphenylmethanol (reference "ref") A. B. C. D.
Expert Answer
The given TLC plate has two spots which help analyse the compounds' Rf value.With the help of Rf of these compounds, we can contrast on the polaritie