Home /
Expert Answers /
Chemistry /
write-the-chemical-reaction-for-hydrogen-thiocyanate-in-water-whose-equilibrium-constant-is-k-pa938
(Solved): Write the chemical reaction for hydrogen thiocyanate in water, whose equilibrium constant is \( K_{ ...
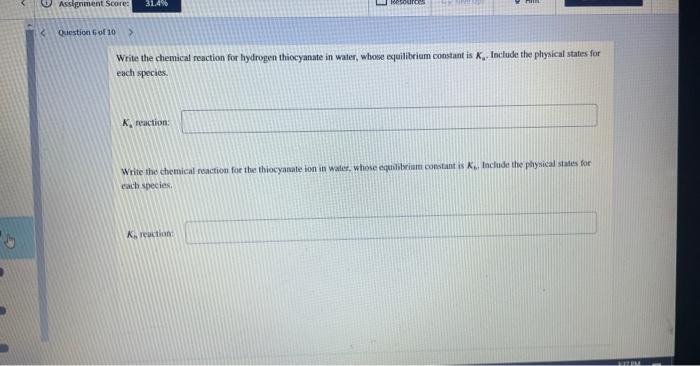
Write the chemical reaction for hydrogen thiocyanate in water, whose equilibrium constant is \( K_{\mathrm{a}} \). Include the plysical states for each species. \( \mathrm{K}_{\mathrm{a}} \) ruaction: Write the chemical reaction foe the thiocyanate ion in walce, whose equililyiart conotant is \( \mathcal{R}_{\text {b. }} \) fachude the pay sical states for cach species. \( \mathrm{K}_{n} \) reuction:
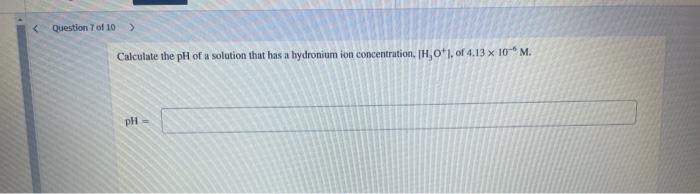
Calculate the \( \mathrm{pH} \) of a solution that has a hydronium ion concentration, \( \left[\mathrm{H}_{3} \mathrm{O}^{+} \mathrm{J}\right. \), of \( 4.13 \times 10^{-6} \mathrm{M} \).