Home /
Expert Answers /
Chemistry /
write-a-balanced-net-ionic-equation-to-show-why-the-solubility-of-baf2-s-increases-in-the-presenc-pa317
(Solved): Write a balanced net ionic equation to show why the solubility of BaF2(s) increases in the presenc ...
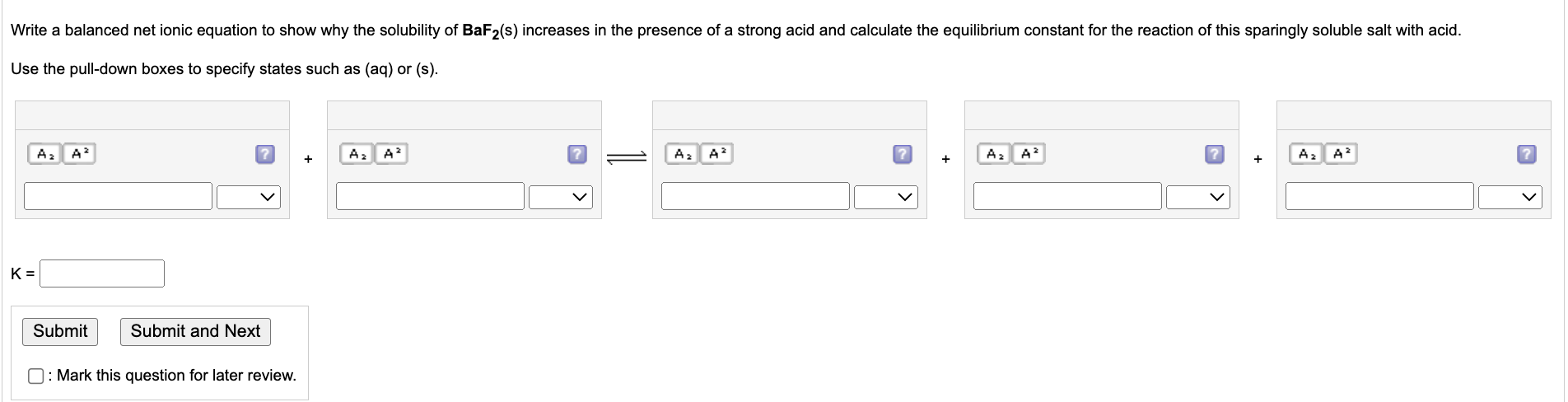
Write a balanced net ionic equation to show why the solubility of BaF2(s) increases in the presence of a strong acid and calculate the equilibrium constant for the reaction of this sparingly soluble salt with acid. Use the pull-down boxes to specify states such as (aq) or (s). K= A? A² Submit Submit and Next : Mark this question for later review. + A? A A? A² + A? A² ? + A? A² ?