Home /
Expert Answers /
Chemistry /
write-a-balanced-equation-for-these-redox-reactions-a-the-oxidation-of-nitrite-ion-to-nitrate-i-pa541
(Solved): Write a balanced equation for these redox reactions. (a) The oxidation of nitrite ion to nitrate i ...
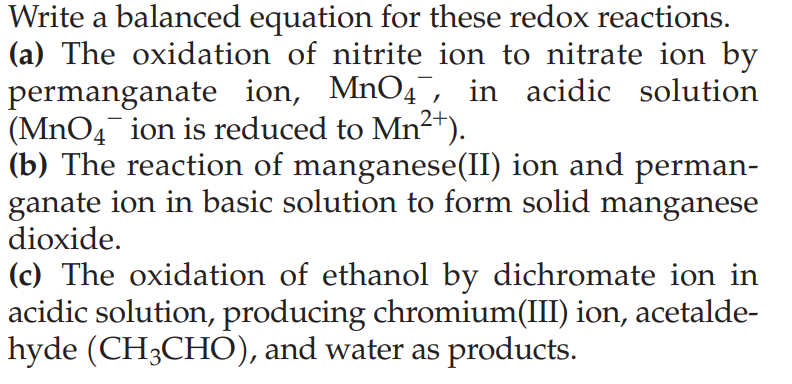
Write a balanced equation for these redox reactions. (a) The oxidation of nitrite ion to nitrate ion by permanganate ion, \( \mathrm{MnO}_{4}^{-} \), in acidic solution \( \left(\mathrm{MnO}_{4}{ }^{-}\right. \)ion is reduced to \( \left.\mathrm{Mn}^{2+}\right) \). (b) The reaction of manganese(II) ion and permanganate ion in basic solution to form solid manganese dioxide. (c) The oxidation of ethanol by dichromate ion in acidic solution, producing chromium(III) ion, acetaldehyde \( \left(\mathrm{CH}_{3} \mathrm{CHO}\right) \), and water as products.