Home /
Expert Answers /
Chemistry /
write-a-balanced-chemical-equation-for-the-decomposition-reaction-do-not-include-phases-part-2-1-pa393
(Solved): Write a balanced chemical equation for the decomposition reaction. Do not include phases. Part 2 (1 ...
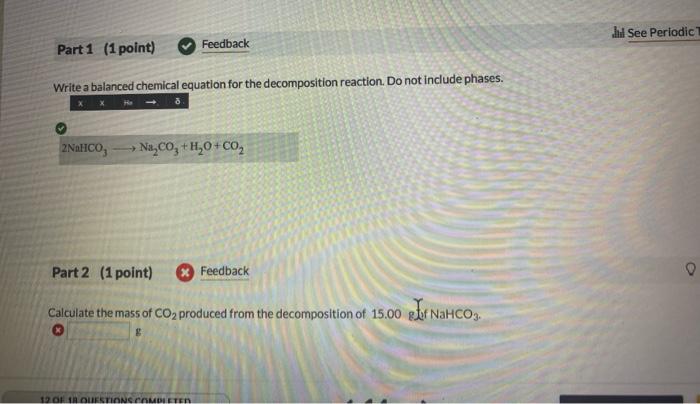
Write a balanced chemical equation for the decomposition reaction. Do not include phases. Part 2 (1 point) Feedback Calculate the mass of produced from the decomposition of g gef .
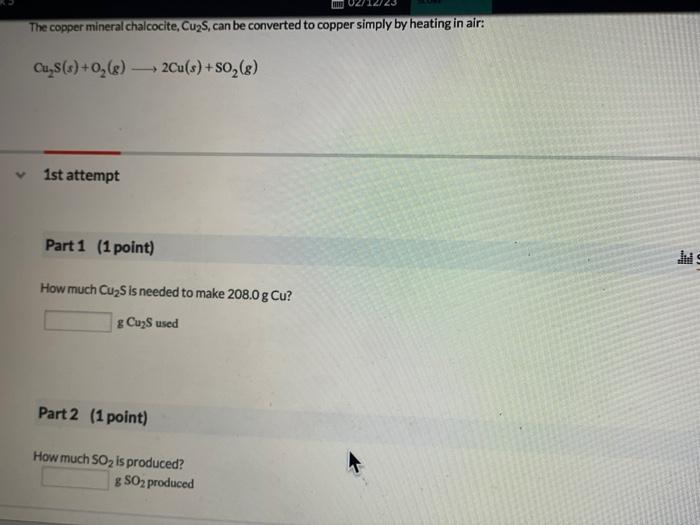
The copper mineral chalcocite, , can be converted to copper simply by heating in air: 1st attempt Part 1 (1 point) How much is needed to make ? used
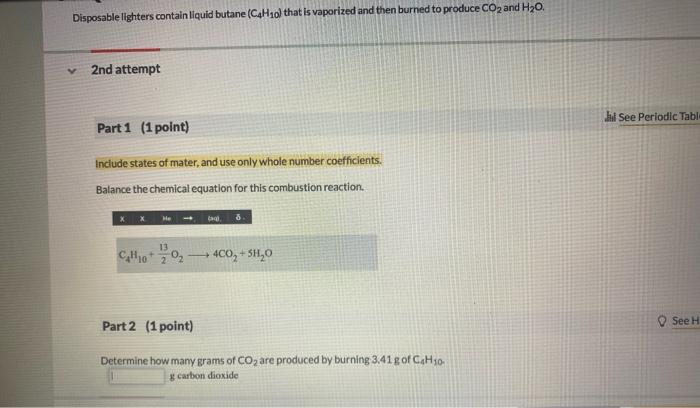
Disposable lighters contain liquid butane o) that is vaporized and then burned to produce and . 2nd attempt Part 1 (1 point) Include states of mater, and use only whole number coefficients. Balance the chemical equation for this combustion reaction. Part 2 (1 point) Determine how many grams of are produced by burning of g carbon dioxide
Expert Answer
2NaHCOA3?NaA2COA3A+HA2OA+COA2from the reaction we can see , 2 moles of NaHCO3 produces 1 mole of CO2mole=mass/molar massmolar mass of NaHCO3 = 84 g/mo