Home /
Expert Answers /
Chemistry /
why-is-the-trans-unsaturated-ketone-obtained-in-this-reaction-instead-of-the-cis-isomer-r-n-r-pa790
(Solved): Why is the trans , - unsaturated ketone obtained in this reaction instead of the cis isomer? \r\n\r\ ...
Why is the trans ,
- unsaturated ketone obtained in this reaction instead of the cis isomer?
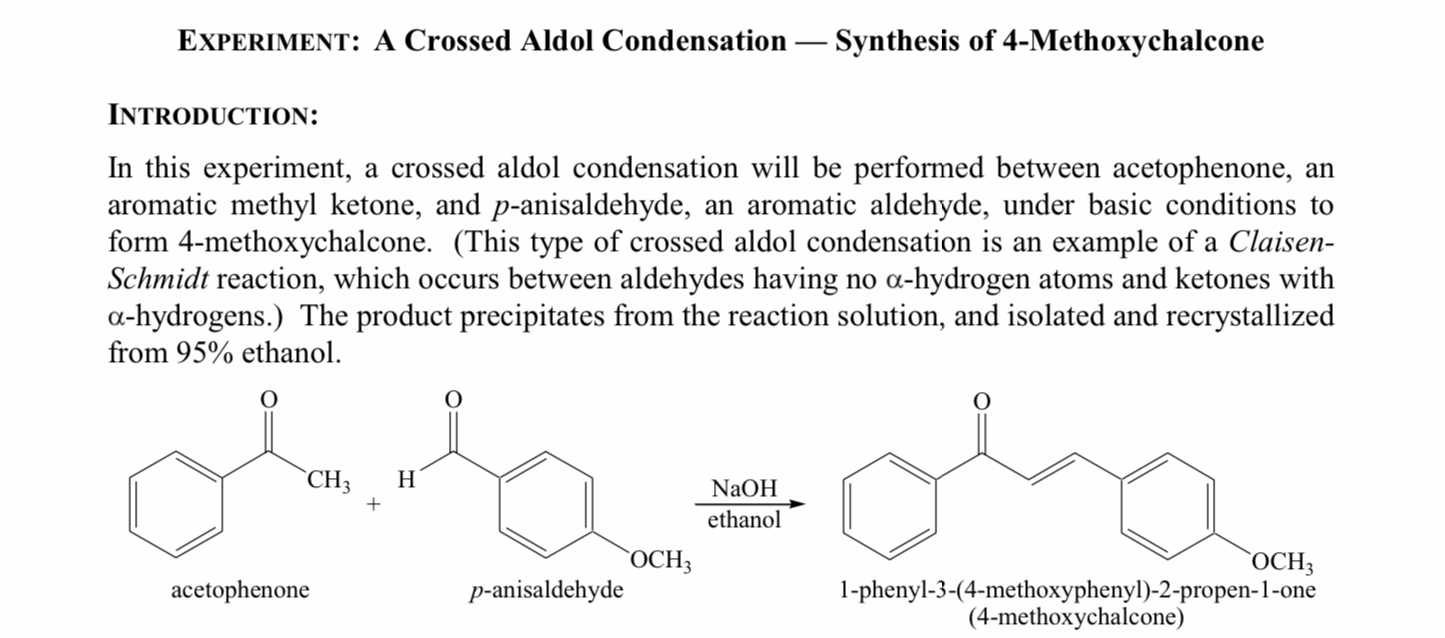
\r\n\r\nINTRODUCTION: In this experiment, a crossed aldol condensation will be performed between acetophenone, an aromatic methyl ketone, and \\( p \\)-anisaldehyde, an aromatic aldehyde, under basic conditions to form 4-methoxychalcone. (This type of crossed aldol condensation is an example of a ClaisenSchmidt reaction, which occurs between aldehydes having no \\( \\alpha \\)-hydrogen atoms and ketones with \\( \\alpha \\)-hydrogens.) The product precipitates from the reaction solution, and isolated and recrystallized from \ ethanol. acetophenone \\( p \\)-anisaldehyde \\[ \\underset{\\text { ethanol }}{\\stackrel{\\mathrm{NaOH}}{\\longrightarrow}} \\] 1-phenyl-3-(4-methoxyphenyl)-2-propen-1-one (4-methoxychalcone)