Home /
Expert Answers /
Chemistry /
which-step-in-this-reaction-mechanism-for-the-iodination-of-acetone-is-the-rate-determining-step-and-pa319
(Solved): Which step in this reaction mechanism for the iodination of acetone is the rate determining step and ...
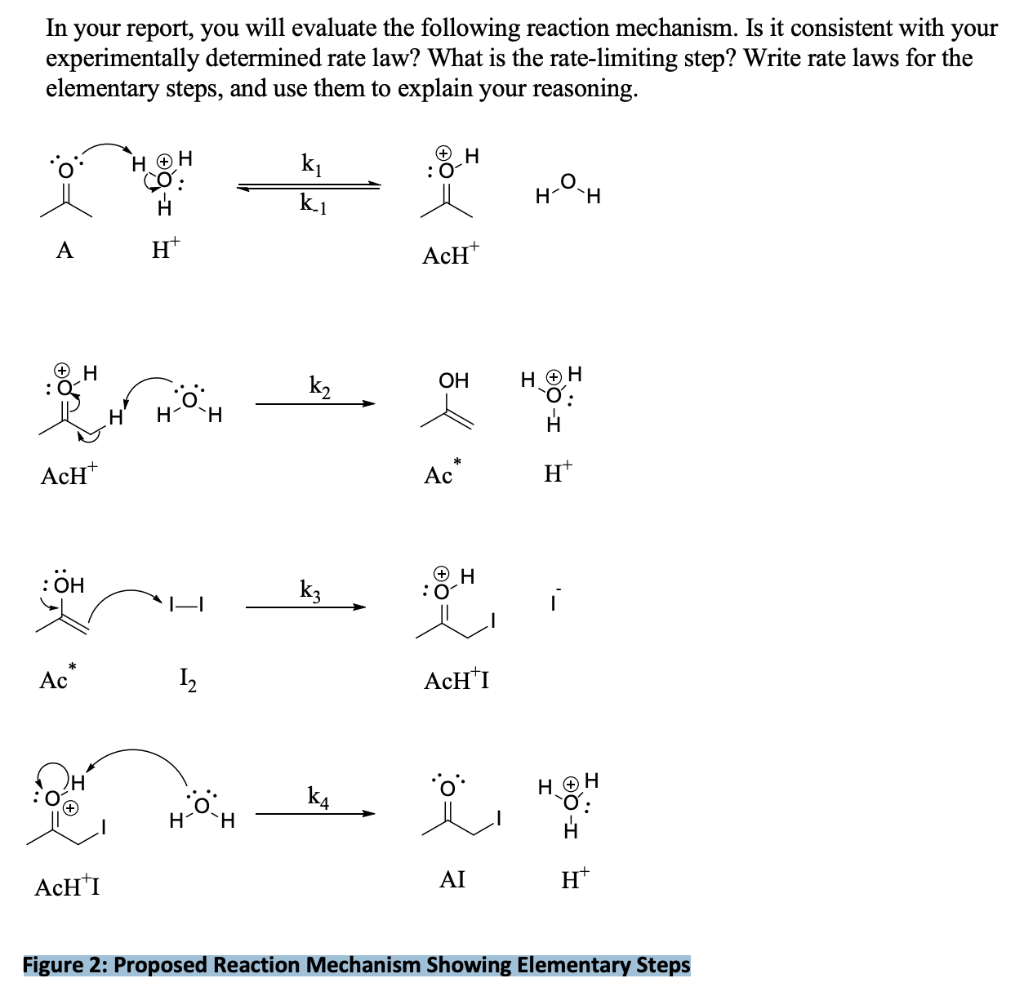
In your report, you will evaluate the following reaction mechanism. Is it consistent with your experimentally determined rate law? What is the rate-limiting step? Write rate laws for the elementary steps, and use them to explain your reasoning. A \( \quad \mathrm{H}^{+} \) \( \mathrm{AcH}^{+} \) \( \mathrm{Ac}^{*} \quad \mathrm{H}^{+} \) \( \mathrm{Ac}^{*} \quad \mathrm{I}_{2} \quad \mathrm{AcH}^{+} \mathrm{I} \) \( \mathrm{AcH}^{+} \mathrm{I} \) AI \( \quad \mathrm{H}^{+} \) Figure 2: Proposed Reaction Mechanism Showing Elementary Steps