Home /
Expert Answers /
Chemistry /
which-of-the-reactions-is-a-decomposition-reaction-2-mathrm-c-2-mathrm-h-6-mathrm-g-pa326
(Solved): Which of the reactions is a decomposition reaction? \( 2 \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g}) ...
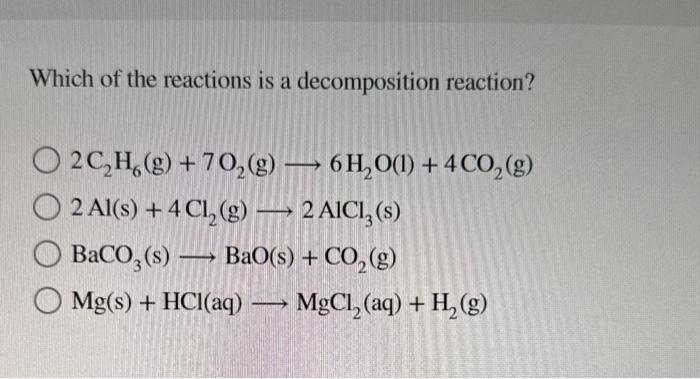
Which of the reactions is a decomposition reaction? \( 2 \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g})+7 \mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 6 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+4 \mathrm{CO}_{2}(\mathrm{~g}) \) \( 2 \mathrm{Al}(\mathrm{s})+4 \mathrm{Cl}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{AlCl}_{3}(\mathrm{~s}) \) \( \mathrm{BaCO}_{3}(\mathrm{~s}) \longrightarrow \mathrm{BaO}(\mathrm{s})+\mathrm{CO}_{2}(\mathrm{~g}) \) \( \mathrm{Mg}(\mathrm{s})+\mathrm{HCl}(\mathrm{aq}) \longrightarrow \mathrm{MgCl}_{2}(\mathrm{aq})+\mathrm{H}_{2}(\mathrm{~g}) \)