Home /
Expert Answers /
Chemistry /
which-of-the-acid-base-reactions-below-would-be-product-favoured-select-one-or-more-begin-arr-pa906
(Solved): Which of the acid-base reactions below would be product-favoured? Select one or more: \[ \begin{arr ...
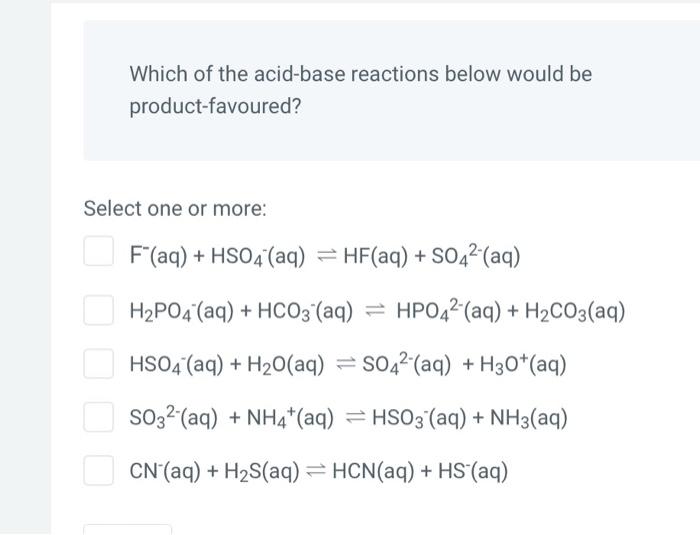
Which of the acid-base reactions below would be product-favoured? Select one or more: \[ \begin{array}{l} \mathrm{F}^{-}(\mathrm{aq})+\mathrm{HSO}_{4}^{-}(\mathrm{aq}) \rightleftharpoons \mathrm{HF}(\mathrm{aq})+\mathrm{SO}_{4}{ }^{2-}(\mathrm{aq}) \\ \left.\mathrm{H}_{2} \mathrm{PO}_{4}^{-}(\mathrm{aq})+\mathrm{HCO}_{3}-\mathrm{aq}\right) \rightleftharpoons \mathrm{HPO}_{4}{ }^{2-}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{CO}_{3}(\mathrm{aq}) \\ \mathrm{HSO}_{4}{ }^{-}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{aq}) \rightleftharpoons \mathrm{SO}_{4}{ }^{2-}(\mathrm{aq})+\mathrm{H}_{3} \mathrm{O}^{+}(\mathrm{aq}) \\ \mathrm{SO}_{3}{ }^{2-}(\mathrm{aq})+\mathrm{NH}_{4}{ }^{+}(\mathrm{aq}) \rightleftharpoons \mathrm{HSO}_{3}{ }^{-}(\mathrm{aq})+\mathrm{NH}_{3}(\mathrm{aq}) \\ \mathrm{CN}^{-}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{~S}(\mathrm{aq}) \rightleftharpoons \mathrm{HCN}(\mathrm{aq})+\mathrm{HS}^{-}(\mathrm{aq}) \end{array} \]
Expert Answer
We know in a Bronsted lowry acid base reaction- an Acid loses H+ ion in solution and thus forms its conjugate base a Base gains this H+ ion in solution and thus forms its conjugate acid. Now a reaction is product favored when the reacti