Home /
Expert Answers /
Chemistry /
which-ground-state-atom-has-an-electron-configuration-described-by-the-following-orbital-diagram-pa635
(Solved): Which ground-state atom has an electron configuration described by the following orbital diagram? \ ...
![Which ground-state atom has an electron configuration described by the following orbital diagram?
\[
[\mathrm{Ne}] \frac{\upa](https://media.cheggcdn.com/study/02a/02a8c5a6-ec27-49b2-bf14-25f5c24d01e3/image)
Which ground-state atom has an electron configuration described by the following orbital diagram? \[ [\mathrm{Ne}] \frac{\uparrow \downarrow}{3 \mathrm{~s}} \quad \uparrow \frac{\uparrow}{3 \mathrm{p}} \stackrel{\uparrow}{1} \] phosphorus nitrogen none of these arsenic
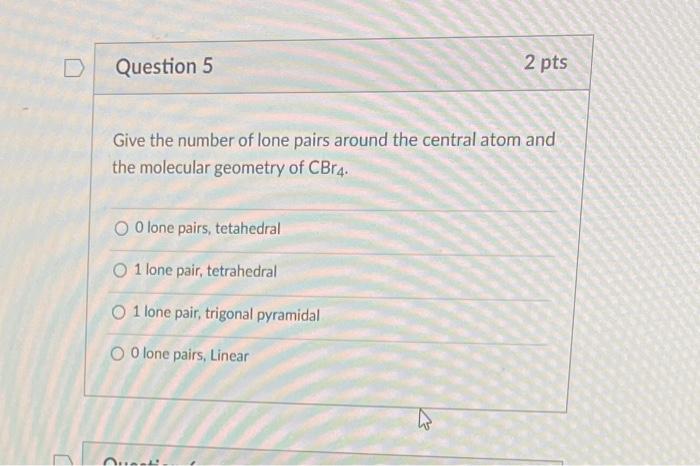
Give the number of lone pairs around the central atom and the molecular geometry of \( \mathrm{CBr}_{4} \). O lone pairs, tetahedral 1 lone pair, tetrahedral 1 lone pair, trigonal pyramidal 0 lone pairs, Linear
Expert Answer
Q. 4. In Ne electron presents= 10 Therefore total electron of the above electron configuration is (10+2+3) =15. Now, among the above