Home /
Expert Answers /
Chemistry /
when-the-mathrm-ag-concentration-is-7-95-times-10-4-mathrm-m-the-observe-pa786
(Solved): When the \( \mathrm{Ag}^{+} \)concentration is \( 7.95 \times 10^{-4} \mathrm{M} \), the observe ...
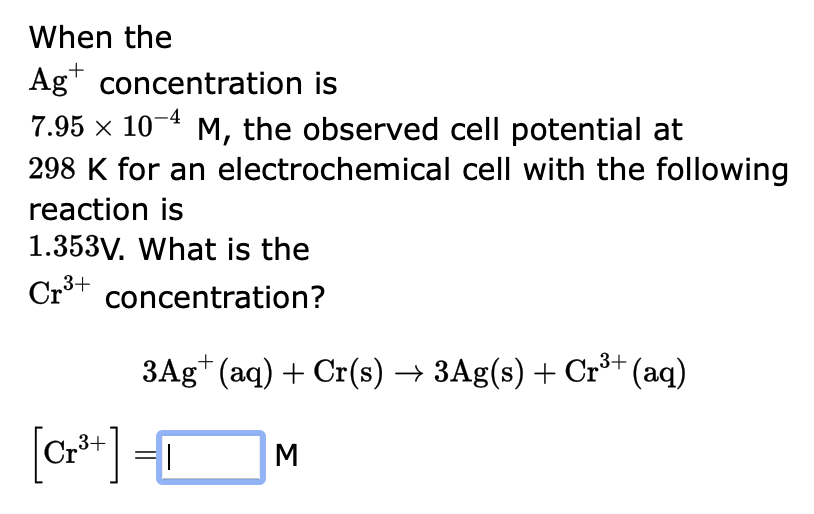
When the \( \mathrm{Ag}^{+} \)concentration is \( 7.95 \times 10^{-4} \mathrm{M} \), the observed cell potential at \( 298 \mathrm{~K} \) for an electrochemical cell with the following reaction is \( 1.353 \mathrm{~V} \). What is the \( \mathrm{Cr}^{3+} \) concentration? \[ \begin{array}{l} \quad 3 \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cr}(\mathrm{s}) \rightarrow 3 \mathrm{Ag}(\mathrm{s})+\mathrm{Cr}^{3+}(\mathrm{aq}) \\ {\left[\mathrm{Cr}^{3+}\right]=\quad \text { M }} \end{array} \]
When the \( \mathrm{Ag}^{+} \)concentration is \( 1.22 \mathrm{M} \), the observed cell potential at \( 298 \mathrm{~K} \) for an electrochemical cell with the following reaction is \( 1.615 \mathrm{~V} \). What is the \( \mathrm{Cr}^{3+} \) concentration? \[ \begin{array}{l} \quad 3 \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cr}(\mathrm{s}) \rightarrow 3 \mathrm{Ag}(\mathrm{s})+\mathrm{Cr}^{3+}(\mathrm{aq}) \\ {\left[\mathrm{Cr}^{3+}\right]=\quad \mathrm{M}} \end{array} \]