Home /
Expert Answers /
Chemistry /
when-the-following-optically-active-alcohol-is-treated-with-hbr-a-racemic-mixture-of-alkyl-bromide-pa787
(Solved): When the following optically active alcohol is treated with HBr, a racemic mixture of alkyl bromide ...


When the following optically active alcohol is treated with HBr, a racemic mixture of alkyl bromides is obtained: HB + H?O Racemic mixture Draw the mechanism for this process, and explain the stereochemical outcome. Which of the following describes the overall mechanism? O Formation of a good leaving group, followed by an S, 1 mechanism. O Formation of a good leaving group, followed by an 5,2 mechanism. O E1 dehydration, followed by an S,,1 mechanism. O E1 dehydration, followed by an S,2 mechanism.
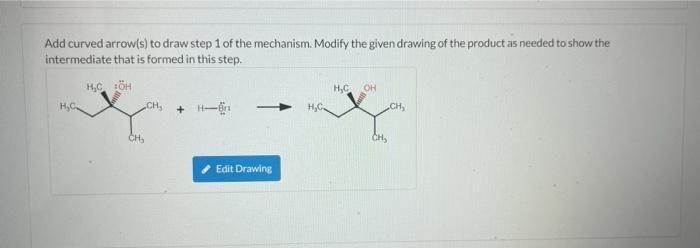
Add curved arrow(s) to draw step 1 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. H?C ÖH H?C OH H?C. CH? CH? Edit Drawing CH? CH?
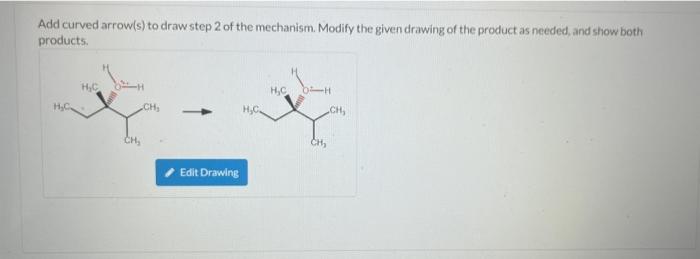
Add curved arrow(s) to draw step 2 of the mechanism. Modify the given drawing of the product as needed, and show both products. H?C H?C H H?C CH? 1 Edit Drawing H?C. CH? CH?
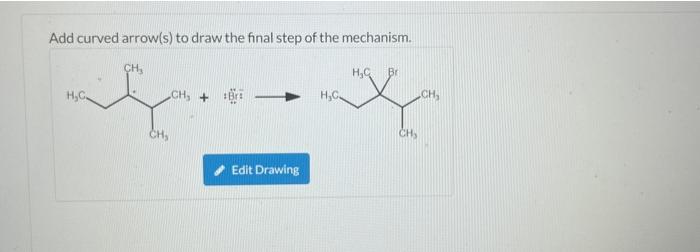
Add curved arrow(s) to draw the final step of the mechanism. CH? Br H?C. CH? + H?C CH? Edit Drawing CH? CH?
Explain the stereochemical outcome. O The alcohol substrate was a racemic mixture. O Protonation of the alcohol gives a racemic carbocation. O Nucleophilic attack occurs at either face of the planar carbocation. O Backside attack results in the formation of both enantiomers.