Home /
Expert Answers /
Chemistry /
when-heated-lithium-reacts-with-nitrogen-to-form-lithium-nitride-6li-s-n2-g-2li3n-s-pa838
(Solved): When heated, lithium reacts with nitrogen to form lithium nitride: 6Li(s)+N2(g)2Li3N(s) ...
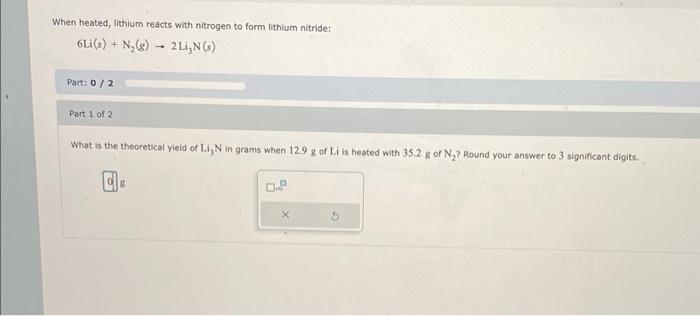
When heated, lithium reacts with nitrogen to form lithium nitride: Part: Part 1 of 2 What is the theoretical yield of in grams when of is heated with of ? Round your answer to 3 significant digits.
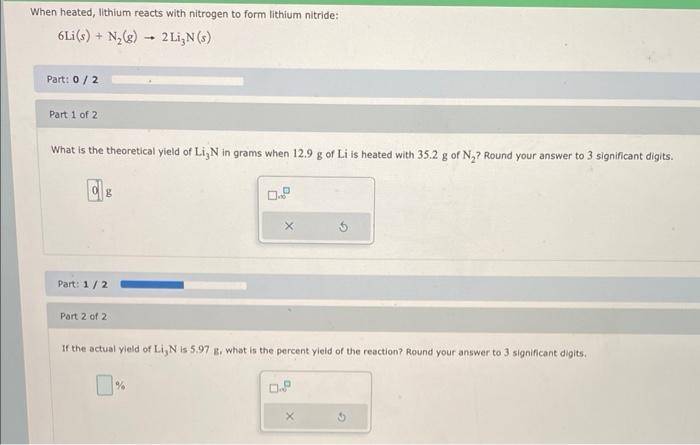
When heated, lithium reacts with nitrogen to form lithium nitride: Part: Part 1 of 2 What is the theoretical yield of in grams when of is heated with of ? Round your answer to 3 significant digits. Part: Part 2 of 2 If the actual yield of is , what is the percent yield of the reaction? Round your answer to 3 significant digits.
Expert Answer
Part 1Given reaction 6Li(s) + N2(g) 2Li3N(s)given dataMass of Lithium =12.9 gMass of Nitrogen gas = 35.2 gfirst, we have to calculate moles using molar mass and atomic mass The atomic mass of Li = The molar mass of N2 gas = Moles can be calculated by using formula, moles of molecule = , Moles of atom = Moles of Li = = Moles of N2 = =