Home /
Expert Answers /
Chemistry /
when-a-certain-weak-field-ligand-forms-an-octahedral-complex-with-mathrm-cd-2-cation-t-pa339
(Solved): When a certain weak-field ligand forms an octahedral complex with \( \mathrm{Cd}^{2+} \) cation, t ...
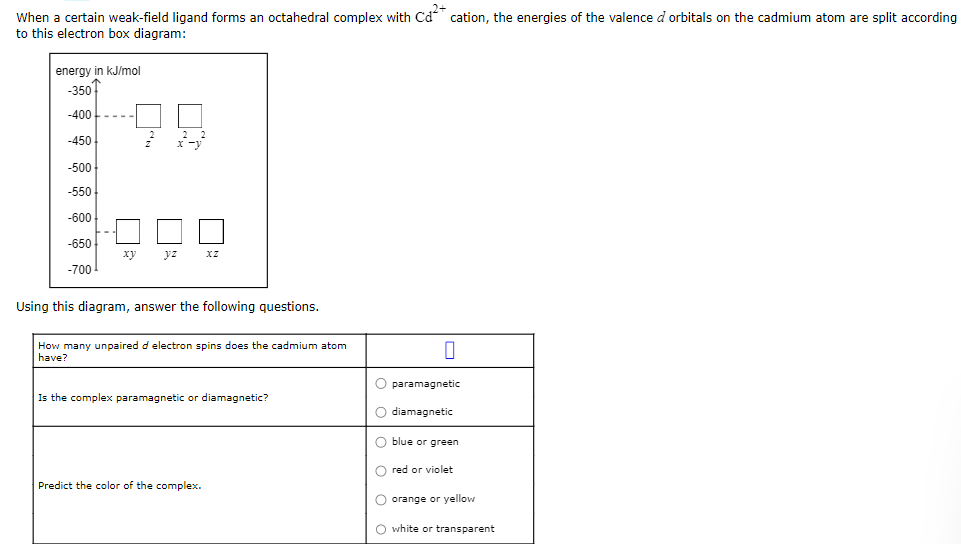
When a certain weak-field ligand forms an octahedral complex with \( \mathrm{Cd}^{2+} \) cation, the energies of the valence \( d \) orbitals on the cadmium atom are split according to this electron box diagram: Using this diagram, answer the following questions.