Home /
Expert Answers /
Chemistry /
what-ratio-of-reactants-cyclohexane-and-chlorine-would-you-use-for-the-synthesis-of-chlorocyclohe-pa249
(Solved): What ratio of reactants (cyclohexane and chlorine) would you use for the synthesis of chlorocyclohe ...
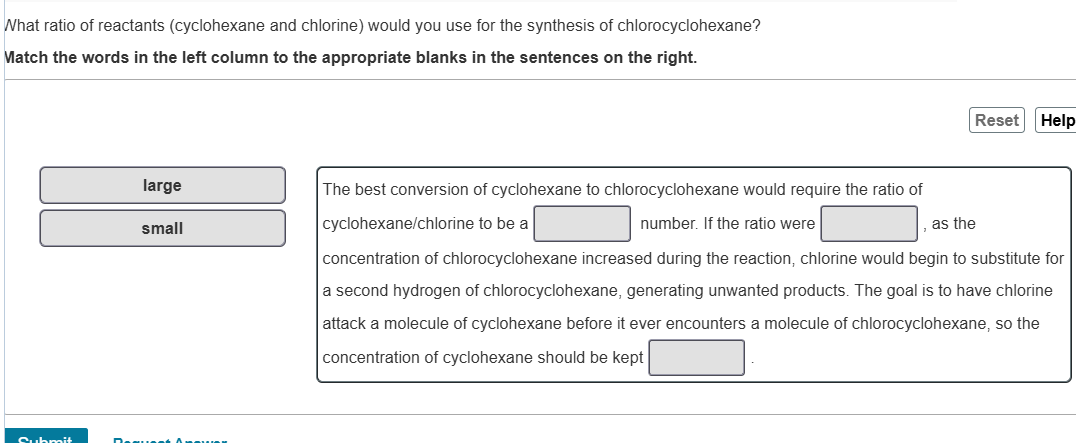
What ratio of reactants (cyclohexane and chlorine) would you use for the synthesis of chlorocyclohexane? Match the words in the left column to the appropriate blanks in the sentences on the right. large small The best conversion of cyclohexane to chlorocyclohexane would require the ratio of cyclohexane/chlorine to be a number. If the ratio were , as the concentration of chlorocyclohexane increased during the reaction, chlorine would begin to substitute for a second hydrogen of chlorocyclohexane, generating unwanted products. The goal is to have chlorine attack a molecule of cyclohexane before it ever encounters a molecule of chlorocyclohexane, so the concentration of cyclohexane should be kept