Home /
Expert Answers /
Chemistry /
what-oxidation-half-reaction-happens-when-iron-reacts-with-hydrochloric-acid-a-2-mathrm-cl-pa371
(Solved): What oxidation half-reaction happens when iron reacts with hydrochloric acid? A. \( 2 \mathrm{Cl}^{ ...
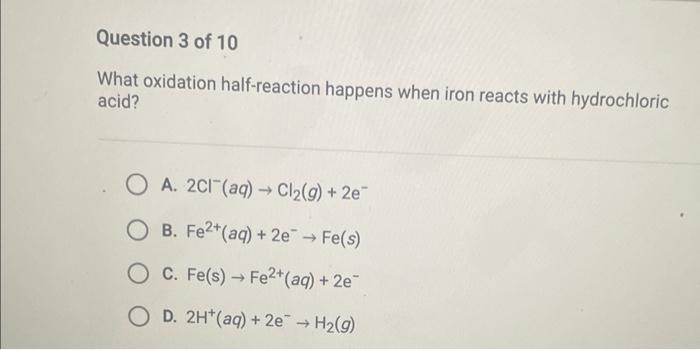
What oxidation half-reaction happens when iron reacts with hydrochloric acid? A. \( 2 \mathrm{Cl}^{-}(a q) \rightarrow \mathrm{Cl}_{2}(g)+2 e^{-} \) B. \( \mathrm{Fe}^{2+}(a q)+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe}(s) \) C. \( \mathrm{Fe}(\mathrm{s}) \rightarrow \mathrm{Fe}^{2+}(a q)+2 \mathrm{e}^{-} \) D. \( 2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}(g) \)
Expert Answer
The correct option is option C Fe(s) --> Fe2+(aq) + 2e- All the other opt