Home /
Expert Answers /
Chemistry /
what-is-wrong-with-the-lewis-dot-structure-shown-below-h-c-n-a-hydrogen-has-two-bonds-four-pa680
(Solved): What is wrong with the Lewis Dot structure shown below? H=C=N: A) Hydrogen has two bonds (four ...
![What is wrong with the Lewis Dot structure shown below?
\[
\mathrm{H}=\mathrm{C}=\mathrm{N} \text { : }
\]
A) Hydrogen has tw](https://media.cheggcdn.com/study/d6d/d6d5228d-3623-4faa-a480-2b0d9d01ecbf/image)


What is wrong with the Lewis Dot structure shown below? A) Hydrogen has two bonds (four electrons). B) Nitrogen atom has only six electrons and not eight. C) Carbon atom has four bonds (eight electrons).
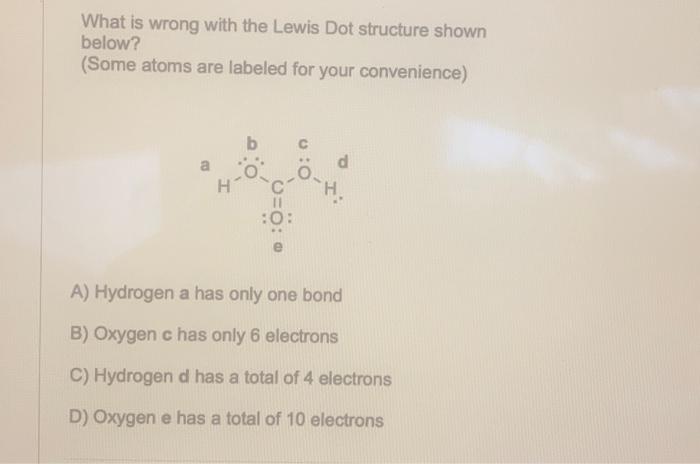
What is wrong with the Lewis Dot structure shown below? (Some atoms are labeled for your convenience) A) Hydrogen a has only one bond B) Oxygen c has only 6 electrons C) Hydrogen d has a total of 4 electrons D) Oxygen e has a total of 10 electrons
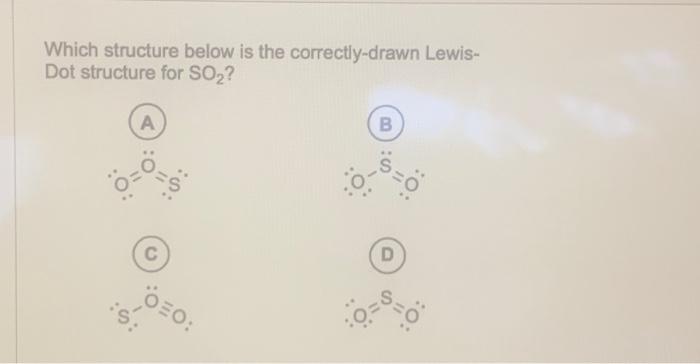
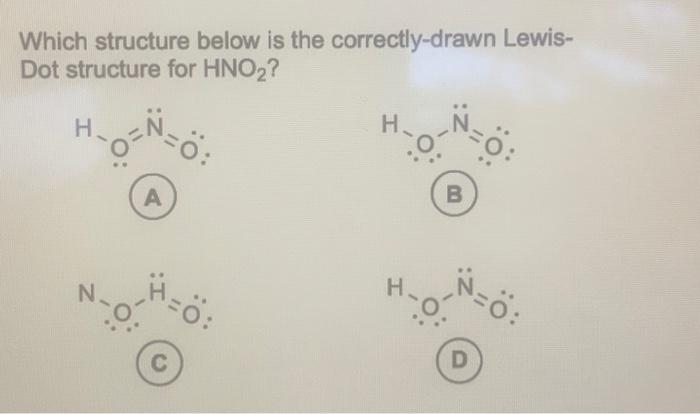
Which structure below is the correctly-drawn LewisDot structure for ?
Whinh otreintium holaus in than anmeantl., dmanum 1 ausia
Which structure below is the correctly-drawn LewisDot structure for ? A) B
Which two atoms follow the "Duet" rule? and H and C and and
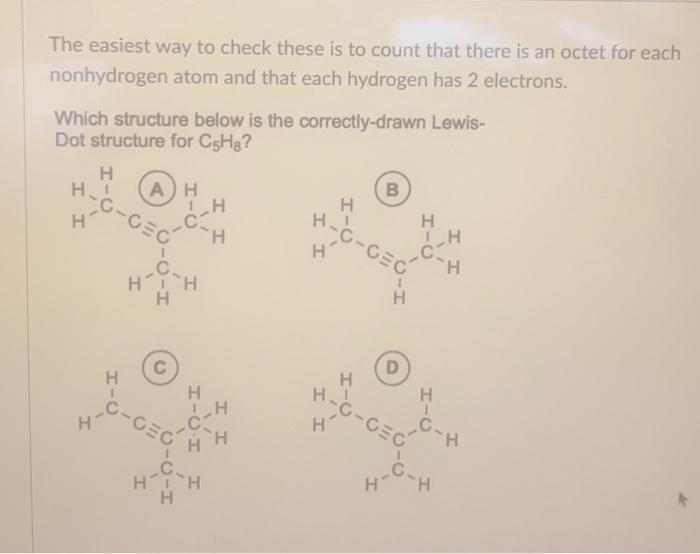
The easiest way to check these is to count that there is an octet for each nonhydrogen atom and that each hydrogen has 2 electrons. Which structure below is the correctly-drawn LewisDot structure for ?
Expert Answer
1 Ans- For the molecule of , we see that ,(a) is incorrect as H cannot have two bonds (four electr...