Home /
Expert Answers /
Chemistry /
what-is-the-value-of-n-in-the-nernst-equation-for-the-balanced-redox-reaction-below-hint-pa912
(Solved): What is the value of \( n \) in the Nernst equation for the balanced redox reaction below? (Hint: ...
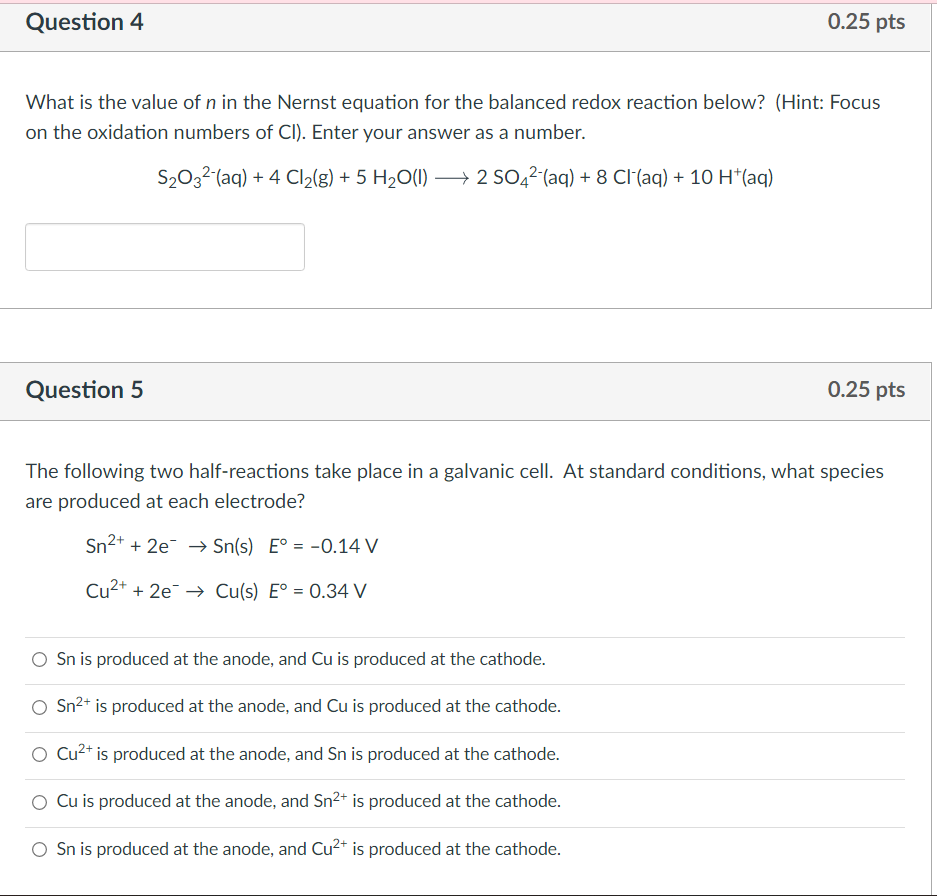
What is the value of \( n \) in the Nernst equation for the balanced redox reaction below? (Hint: Focus on the oxidation numbers of \( \mathrm{Cl} \) ). Enter your answer as a number. \[ \mathrm{S}_{2} \mathrm{O}_{3}{ }^{2-}(\mathrm{aq})+4 \mathrm{Cl}_{2}(\mathrm{~g})+5 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \longrightarrow 2 \mathrm{SO}_{4}{ }^{2-}(\mathrm{aq})+8 \mathrm{Cl}^{-}(\mathrm{aq})+10 \mathrm{H}^{+}(\mathrm{aq}) \] Question 5 \( 0.25 \) pts The following two half-reactions take place in a galvanic cell. At standard conditions, what species are produced at each electrode? \[ \begin{array}{l} \mathrm{Sn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn}(\mathrm{s}) \quad \mathrm{E}^{\circ}=-0.14 \mathrm{~V} \\ \mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s}) \mathrm{E}^{\circ}=0.34 \mathrm{~V} \end{array} \] Sn is produced at the anode, and \( \mathrm{Cu} \) is produced at the cathode. \( \mathrm{Sn}^{2+} \) is produced at the anode, and \( \mathrm{Cu} \) is produced at the cathode. \( \mathrm{Cu}^{2+} \) is produced at the anode, and \( \mathrm{Sn} \) is produced at the cathode. \( \mathrm{Cu} \) is produced at the anode, and \( \mathrm{Sn}^{2+} \) is produced at the cathode. \( \mathrm{S} n \) is produced at the anode, and \( \mathrm{Cu}^{2+} \) is produced at the cathode.