Home /
Expert Answers /
Chemistry /
what-is-the-strongest-type-of-intermolecular-force-in-the-following-compounds-ch3ch2nh2-0-ch3n-pa224
(Solved): What is the strongest type of intermolecular force in the following compounds? CH3CH2NH2 0 CH3N ...
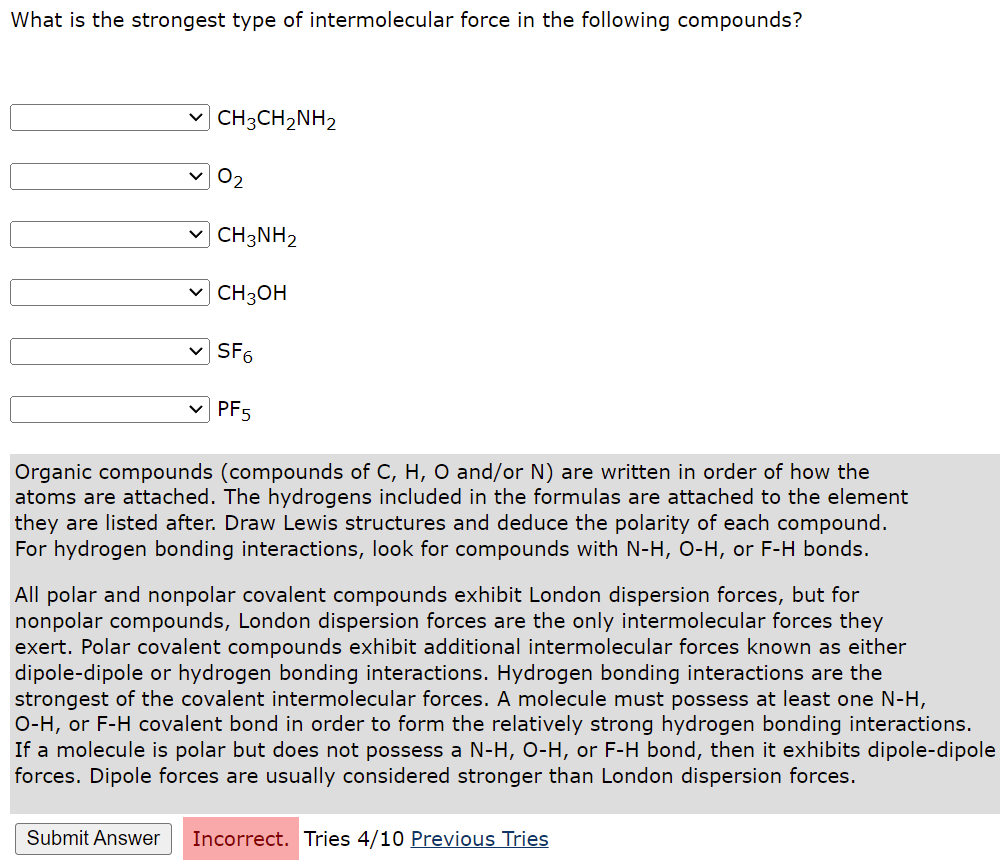
What is the strongest type of intermolecular force in the following compounds? CH3CH2NH2 0? CH3NH2 CH3OH ?SF6 PF5 Organic compounds (compounds of C, H, O and/or N) are written in order of how the atoms are attached. The hydrogens included in the formulas are attached to the element they are listed after. Draw Lewis structures and deduce the polarity of each compound. For hydrogen bonding interactions, look for compounds with N-H, O-H, or F-H bonds. All polar and nonpolar covalent compounds exhibit London dispersion forces, but for nonpolar compounds, London dispersion forces are the only intermolecular forces they exert. Polar covalent compounds exhibit additional intermolecular forces known as either dipole-dipole or hydrogen bonding interactions. Hydrogen bonding interactions are the strongest of the covalent intermolecular forces. A molecule must possess at least one N-H, O-H, or F-H covalent bond in order to form the relatively strong hydrogen bonding interactions. If a molecule is polar but does not possess a N-H, O-H, or F-H bond, then it exhibits dipole-dipole forces. Dipole forces are usually considered stronger than London dispersion forces. Submit Answer Incorrect. Tries 4/10 Previous Tries