Home /
Expert Answers /
Chemistry /
what-is-the-reason-for-calibrating-the-uv-spectrophotometer-with-distilled-water-to-check-if-the-pa627
(Solved): What is the reason for calibrating the UV spectrophotometer with distilled water? To check if the ...
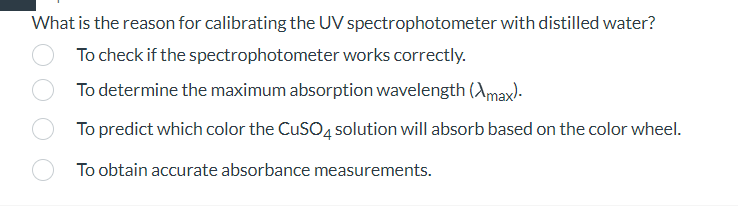
What is the reason for calibrating the UV spectrophotometer with distilled water? To check if the spectrophotometer works correctly. To determine the maximum absorption wavelength . To predict which color the solution will absorb based on the color wheel. To obtain accurate absorbance measurements.
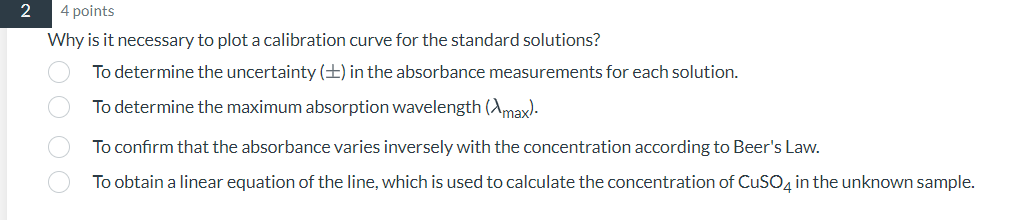
4 points Why is it necessary to plot a calibration curve for the standard solutions? To determine the uncertainty in the absorbance measurements for each solution. To determine the maximum absorption wavelength . To confirm that the absorbance varies inversely with the concentration according to Beer's Law. To obtain a linear equation of the line, which is used to calculate the concentration of in the unknown sample.
2 points From the equation of the line, what two values can be obtained from the slope? Molar absorptivity and path length (I). Absorbance (A) and molar absorptivity . and absorbance. Path length (I) and .
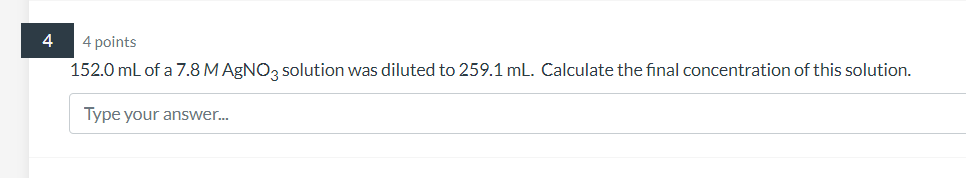
4 points 152.0 mL of a solution was diluted to . Calculate the final concentration of this solution. Type your answer...
4 points A calibration curve gives a slope of . If the path length equals , calculate the molar absorptivity . Round your answer to PLACE. Type your answer...
4 points your answer to 1 DECIMAL PLACE. Type your answer...
Expert Answer
Answer:As per Chegg guidelines, only 1st question can be answered, so for the remaining questions post them separately. The answer to the 1st question