Home /
Expert Answers /
Chemistry /
what-is-the-oxidation-number-of-each-atom-in-sodium-hydrogen-carbonate-nahco3-na-1-h-1-c-pa269
(Solved): What is the oxidation number of each atom in sodium hydrogen carbonate, NaHCO3? Na = +1, H = -1, C ...
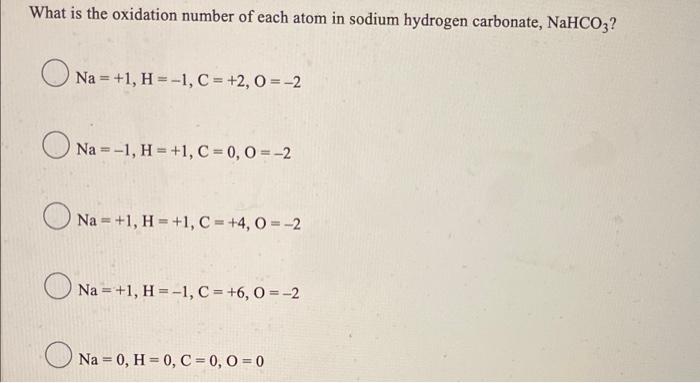
What is the oxidation number of each atom in sodium hydrogen carbonate, NaHCO3? Na = +1, H = -1, C = +2,0 = -2 Na --1, H=+1, C = 0, 0 = -2 Na=+1, H = +1, C = +4,0 = -2 Na=+1, H = -1, C = +6,0 = -2 Na = 0, H = 0, C = 0, 0 = 0
Expert Answer
According to the metal theory metals mostly have a positive oxidation state and since Na belongs to alkali metals, it's ox