Home /
Expert Answers /
Chemistry /
what-is-the-hybridization-state-of-the-nitrogens-in-the-following-molecule-all-three-are-s-p-2-pa791
(Solved): What is the hybridization state of the nitrogens in the following molecule? all three are \( s p^{2 ...
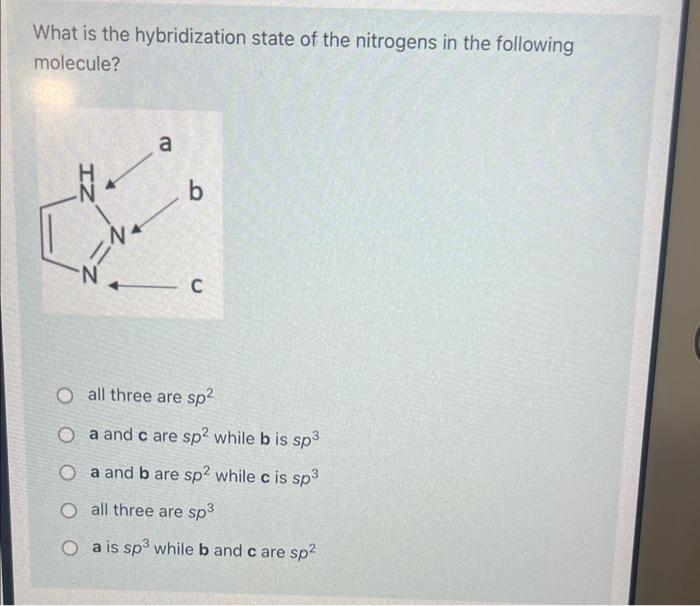
What is the hybridization state of the nitrogens in the following molecule? all three are \( s p^{2} \) a and \( \mathbf{c} \) are \( s p^{2} \) while \( \mathbf{b} \) is \( s p^{3} \) \( \mathbf{a} \) and \( \mathbf{b} \) are \( s p^{2} \) while \( \mathbf{c} \) is \( s p^{3} \) all three are \( s p^{3} \) \( \mathbf{a} \) is \( s p^{3} \) while \( \mathbf{b} \) and \( \mathbf{c} \) are \( s p^{2} \)
Expert Answer
Answer: all three are sp2 Solution: Steric number = number of sigma bonds + number of localised