Home /
Expert Answers /
Chemistry /
what-is-the-correct-equilibrium-constant-expression-for-the-balanced-reaction-shown-below-math-pa724
(Solved): What is the correct equilibrium constant expression for the balanced reaction shown below? \[ \math ...
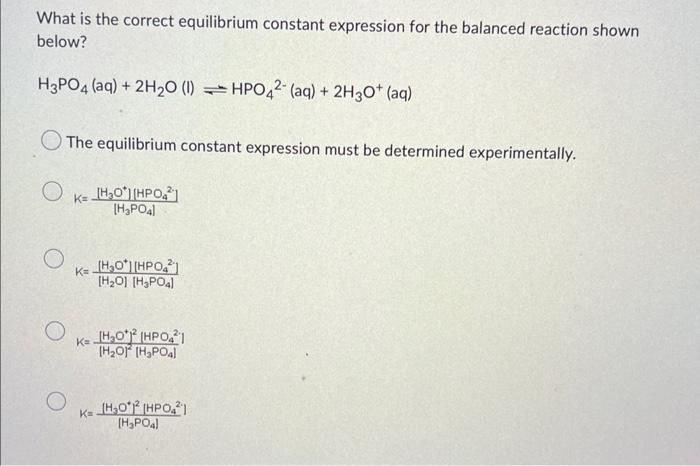
What is the correct equilibrium constant expression for the balanced reaction shown below? \[ \mathrm{H}_{3} \mathrm{PO}_{4}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{HPO}_{4}^{2-}(\mathrm{aq})+2 \mathrm{H}_{3} \mathrm{O}^{+}(\mathrm{aq}) \] The equilibrium constant expression must be determined experimentally. \[ K=\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{HPO}_{4}^{2}\right]}{\left[\mathrm{H}_{3} \mathrm{PO}_{4}\right]} \] \[ K=\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{HPO}_{4}{ }^{2-}\right]}{\left[\mathrm{H}_{2} \mathrm{O}\right]\left[\mathrm{H}_{3} \mathrm{PO}_{4}\right]} \] \[ K=\frac{\left|\mathrm{H}_{3} \mathrm{O}^{+}\right|^{2}\left[\mathrm{HPO}_{4}^{2}\right]}{\left[\mathrm{H}_{3} \mathrm{PO}_{4} \mid\right.} \]