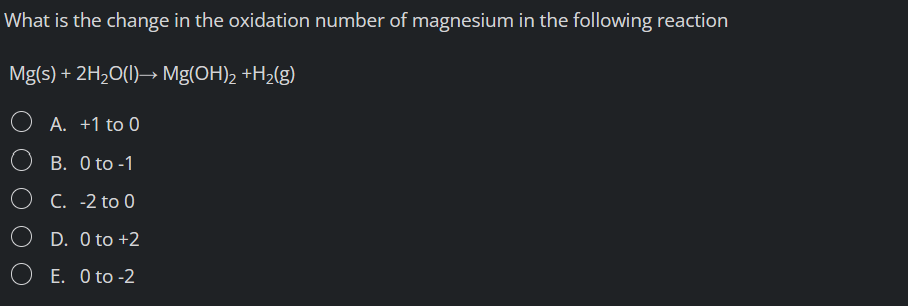Home /
Expert Answers /
Chemistry /
what-is-the-change-in-the-oxidation-number-of-magnesium-in-the-following-reaction-mathrm-mg-pa833
(Solved): What is the change in the oxidation number of magnesium in the following reaction \[ \mathrm{Mg}(\ ...
What is the change in the oxidation number of magnesium in the following reaction \[ \mathrm{Mg}(\mathrm{s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Mg}(\mathrm{OH})_{2}+\mathrm{H}_{2}(\mathrm{~g}) \] A. \( +1 \) to 0 B. 0 to \( -1 \) C. \( -2 \) to 0 D. 0 to \( +2 \) E. 0 to \( -2 \)
Expert Answer
The answer is 0 to +2. The following react