Home /
Expert Answers /
Chemistry /
what-is-the-balanced-equation-for-reaction-betweon-sodium-phosphate-and-ammonium-nitrate-mathr-pa137
(Solved): What is the balanced equation for reaction betweon sodium phosphate and ammonium nitrate? \[ \mathr ...
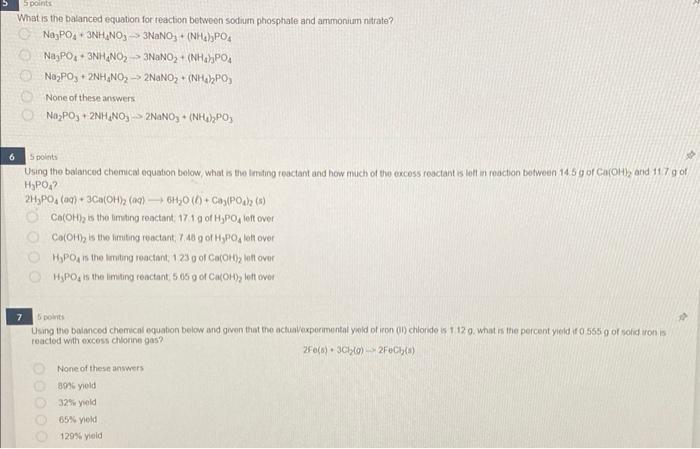
What is the balanced equation for reaction betweon sodium phosphate and ammonium nitrate? \[ \mathrm{Na}_{3} \mathrm{PO}_{4}+3 \mathrm{NH}_{4} \mathrm{NO}_{3} \rightarrow 3 \mathrm{NaNO}_{3}+\left(\mathrm{NH}_{4}\right)_{3} \mathrm{PO}_{4} \] \[ \begin{array}{l} \mathrm{Na}_{3} \mathrm{PO}_{4}+3 \mathrm{NH}_{4} \mathrm{NO}_{2} \rightarrow 3 \mathrm{NaNO}_{2}+\left(\mathrm{NH}_{4} \mathrm{PPO}_{4}\right. \\ \mathrm{Na}_{2} \mathrm{PO}_{3}+2 \mathrm{NH}_{4} \mathrm{NO}_{2} \rightarrow 2 \mathrm{NaNO}_{2}+\left(\mathrm{NH}_{4} 2_{2} \mathrm{PO}_{3}\right. \end{array} \] None of these answers \[ \mathrm{Na}_{2} \mathrm{PO}_{3}+2 \mathrm{NH}_{4} \mathrm{NO}_{3} \rightarrow 2 \mathrm{NaNO}_{3}+\left(\mathrm{NH}_{4}\right)_{2} \mathrm{PO}_{3} \] spolints Using the balanced chemical equation below, what is the lenting reaciant and how misich of tho excoss reactant is loll in reaction botwoen \( 14.5 \mathrm{~g} \) of \( \mathrm{Ca}(\mathrm{OH} \mathrm{h} \) and \( 117 \mathrm{~g} \) of \( \mathrm{H}_{3} \mathrm{PO}_{4} \) ? \[ 2 \mathrm{H}_{2} \mathrm{PO}_{4}(a q)+3 \mathrm{Ca}(\mathrm{OH})_{2}(a q) \rightarrow \mathrm{OH}_{2} \mathrm{O}(b)+\mathrm{Ca}_{2}\left(\mathrm{PO}_{4}\right)_{2} \text { (s) } \] \( \mathrm{Ca}(\mathrm{OH})_{2} \) is the limting reactiont, \( 17.1 \mathrm{~g} \) of \( \mathrm{H}_{3} \mathrm{PO}_{4} \) left over \( \mathrm{Ca}(\mathrm{OH})_{2} \) is the limiteng reactant, \( 7.40 \mathrm{~g} \) of \( \mathrm{H}_{3} \mathrm{PO}_{4} \) loft over \( \mathrm{H}_{3} \mathrm{PO}_{4} \) is the limiting reactant, 123 o of \( \mathrm{Ca}(\mathrm{OH})_{2} \) lott over \( \mathrm{H}_{3} \mathrm{PO}_{4} \) is the limeting reactant, \( 505 \mathrm{gol} \mathrm{Ca}(\mathrm{OH}) \) ) left over 7 Sponts Using the baingcod chemical equation below and given that the actualexpermental yold of iron (il) chloride is 1120 . what is the percent yield if \( 0.555 .9 \) of toid iron is reacied with excess chtorine gas? \[ 2 F 0(0)+3 \mathrm{Cl}_{2}(\theta)-2 F 0 \mathrm{Ch}_{(3)}(3) \] None of these answers ans yoid \( 32 \% \) yold \( 65 \% \) yeld 1295 yiold
Expert Answer
Answer The limiting reagent is the