Home /
Expert Answers /
Biology /
what-does-the-figure-represent-please-summarize-disparity-through-time-plots-using-the-body-size-pa890
(Solved): what does the figure represent? please summarize Disparity-through-time plots using the body size ...
what does the figure represent? please summarize
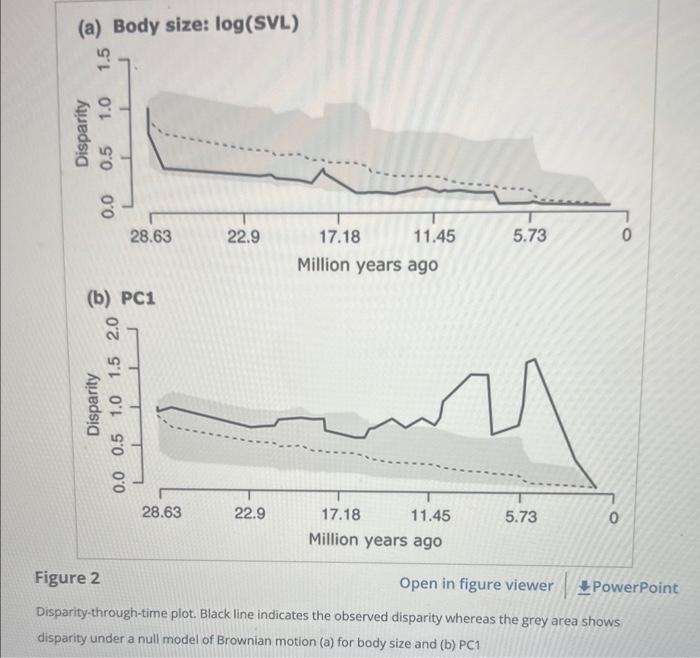






Disparity-through-time plots using the body size data (log of SVL) and PC1 displayed differing results (Figure 2). The average subclade disparity for size was not significantly different from the expectations under the Brownian motion model. On the other hand, PC1 axis showed increased disparity, increasing beyond the null expectation beginning , with considerable increase Mya (Figure 2b). DTT analysis of the size-corrected toepad length and breadth showed identical DTT plot to PC1 (Figure S2), confirming that this increase in disparity is contributed by differences in toepad size. The MDI value for body size was indicating that disparity in body size was partitioned between subclades as compared to , which had a value of .4428, indicating that disparity was partitioned within subclades. However, the -values associated with MDI were not significant ( -value SVL ; ). The maximum likelihood model fit showed that the model fit body size the best, whereas PC1 best fit OU model based on the AICc scores (Table 1). The follow-up MCMC-based simulations showed that the data simulated based on the body size data did not have the statistical power to distinguish between the BM and OU models (randomization test, -value ), whereas for , re-estimated parameter values from simulations under different models were significantly different, showing that model fitting for PC1 was robust (randomization test, -value < .05; Figure ). Blomberg's for body size had a value of (randomization test, -value ) showing phylogenetic signal and lying close to the value expected under BM model. In contrast, PC1 had a value of . 40 with the -value of randomization test being indicating a lack of phylogenetic signal. Blomberg's for toepad variables were similar to ranging between 3 and 6
(a) Body size: (SVL) (b) PC1 Figure 2 Open in figure viewer Disparity-through-time plot. Black line indicates the observed disparity whereas the grey area shows disparity under a null model of Brownian motion (a) for body size and (b)
The "early-burst" model of adaptive radiation predicts an early increase in phenotypic disparity concurrent with lineage diversification. Although most studies report a lack of this coupled pattern, the underlying processes are not identified. The continental radiation of Hemidactylus geckos from Peninsular India includes morphologically diverse species that occupy various microhabitats. This radiation began diversifying 36 Mya with an early increase in lineage diversification. Here, we test the "early-burst" hypothesis by investigating the presence of ecomorphs and examining the pattern of morphological diversification in a phylogenetic framework. Two ecomorphs - terrestrial and scansorial species - that vary significantly in body size and toepad size were identified. Unlike the prediction of the "early-burst" model, we find that disparity in toepad morphology accumulated more recently 14 Mya and fit the Ornstein-Ulhenbeck model. Ancestral state reconstruction of the two ecomorphs demonstrates that terrestrial lineages evolved independently at least five times from scansorial ancestors, with the earliest diversification in terrestrial lineages 19-12 Mya. Our study demonstrates a delayed increase in morphological disparity as a result of the evolution of terrestrial ecomorphs. The diversification of terrestrial lineages is concurrent with the establishment of open habitat and grasslands in Peninsular India, suggesting that the appearance of this novel resource led to the adaptive diversification.
Adaptive radiations are fascinating model systems to examine lineage as well as phenotypic diversification and provide key insights into the tempo and mode of diversification (Harmon et al., 2010: Harmon, Schulte, Larson, \& Losos, 2003). They are often described as rapid dlversification of a single ancestor into multiple ecologically diverse species (Futuyma, 1998; Givnish \& Sytsma, 2000; Schluter, 2000). Fossil evidence and molecular systematics have shown that such spectacular radiations are facilitated by ecological opportunity (Schluter, 2000; Stroud \& Losos, 2016; Wagner, Harmon, \& Seehausen, 2012). This opportunity couid arise as a result of colonization of a landmass or lake devoid of competition, the emergence of a new resource, elimination of antagonists due to extinction events or through the evolution of traits that allow species to exploit a novel environment (Simpson, 1944, 1953). Studies on several radiations have demonstrated that presence of ecological opportunity often results in rapid accumulation of lineages early in the history of the radiation, followed by a "slowing down" of diversification rates later (MCPeek, 2008; Morlon, Potts, \& Plotkin, 2010; Nee, Mooers, \& Harvey, 1992; Phillimore \& Price, 2008). George Gaylord Simpson proposed that ecological opportunity should result in a concurrent burst of lineages as well as phenotypic diversification (Schluter, 2000; Simpson, 1953). However, such association between species diversification and phenotypic diversification is rare (Burbrink, Chen, Myers, Brandiey, \& Pyron, 2012; Harmon et al., 2010; but see Burbrink \& Pyron, 2009; Price, Etienne; 8. Powell, 2016). It is possible that the underlying processes driving these two patterns are disparate in many cases. The theory, as well as empirical data as to why these two patterns are decoupled, needs more attention in the adaptive radiation literature. Ecological opportunity is often thought to underlie exceptional adaptive radiations in insular sustems: however its role in generating adantive diversity at the continental scale is unclear
Continental systems, being far more complex, are likely to have divergent patterns from those of insular systems given the large area, relatively older age and pre-established biota (Derryberry et al., 2011; Liedtke et al., 2016; Losos \& Ricklefs, 2009; Maestri et al., 2016). While some studies point towards the role of ecological opportunity in generating continental adaptive radiations (Barker, Burns, Klicka, Lanyon, \& Lovette, 2013; Burbrink et al. 2012; Burbrink \& Pyron, 2009; Drummond, Eastwood, Miotto, \& Hughes, 2012; McGuire et al., 2014: Price et al., 2014; Schenk, Rowe, \& Steppan, 2013), others do not detect the presence of ecological opportunity (Alhajeri \& Schenk, 2016; Claramunt, Derryberry, Brumfield, \& Remsen, 2012; Day et al., 2013; Derryberry et al., 2011; Liedtke et al., 2016: Schweizer, Hertwig, \& Seehausen, 2014; Tran, 2014). Moreover, the relationship between ecological opportunity and the resulting phenotypic diversifications is varied (Derryberry et al., 2011; Garcia-Porta, Šmid, Sol, Fasola, \& Carranza, 2016; Maestri et al., 2016; Pinto, Mahler, \& Harmon, 2008), with studies reporting constrained phenotypic diversity in continental systems (Derryberry et al., 2011). Peninsular India offers an interesting setting to study adaptive radiations in a continental context, Change in climate due to global as well as regional factors had a profound influence on the Peninsular Indian biota (Agarwal \& Ramakrishnan, 2017; Deepak \& Karanth, 2018; Klaus, Morley, Plath, Zhang, \& Li, 2016; Morley, 2000, 2018) and could have acted as an ecological opportunity facilitating the diversification of numerous taxa. Until the EoceneOligocene transition million years ago (Mya), Peninsular India is known to have harboured rainforest flora. The cooling during the Eocene-Oligocene transition led to a transition from rainforest flora to more dry and seasonal forests (Morley, 2000). A subsequent major shift in the vegetation began Mya, as monsoon seasonality began to intensify (Clift et al., 2008), which was followed by intense aridification in the late Miocene -10 Mya (Molnar \& Rajagopalan, 2012; Nelson, 2007). There was a marked increase in C4 grasses during this period. Subsequently, by 8-6.5 Mya, the northern Indian subcontinent is
intensify (Clift et al., 2008), which was followed by intense aridification in the late Miocene 10 Mya (Molnar \& Rajagopalan, 2012; Nelson, 2007). There was a marked increase in C4 grasses during this period. Subsequently, by 8-6.5 Mya, the northern Indian subcontinent is thought to have been dominated by grassland vegetation (Hoorn, Ohja, \& Quade, 2000). A large part of Peninsular India at present can be categorized as the dry zone (Karanth, 2003), dominated by dry-deciduous forests, scrub and savanna (Morley, 2018). Although little information is available regarding when this biome was established in the rest of the Indian subcontinent, studies on diversification of dry- and open-habitat lizards have shed some light on this. The fan-throated lizards that are widespread across the dry zone of Peninsular India, particularly in open habitats, began diversifying 18 Mya (95\% High posterior density, HPD: 22.9-14.1), with a marked increase in diversification 15-9 Mya (Deepak \& Karanth, 2018). Similarly, a group of lacertid lizards restricted to open habitats began diversifying 30 Mya (95\% HPD: 34-26 Mya), with a shift towards increased diversification Mya (Aganwal \& Ramakrishnan, 2017). Interestingly, a different pattern of diversification is seen in the Indian radiation of Hemidactylus geckos, which are also distributed predominantly in the dry zone of Peninsular India. The Indian radiation of Hemidactylus geckos began diversifying 36 Mya (95\% HPD: 32.3-39.4), concurrent with the Eocene-Oligocene cooling. A recent study by Lajmi and Karanth (2019) revealed an early increase in lineage diversification in this radiation followed by a slowdown. Species within the Indian Hemidactylus radiation can be broadly grouped into scansorial species, that is species that inhabit vertical surfaces, and terrestrial or ground-dwelling species. Although the terrestrial species predominantly inhabit open habitat and grasslands, the scansorial species can be further categorized into rupicolous (inhabiting rocky outcrops), arboreal (inhabiting tree trunks) and human-commensal species. Although species of this group display substantial morphological disparity, whether these morphological differences correspond to ecomorphs is not known.