Home /
Expert Answers /
Chemistry /
we-have-to-use-the-data-in-the-table-to-find-the-concentration-of-iodide-ions-and-sodium-peroxdisul-pa806
(Solved): We have to use the data in the table to find the concentration of Iodide ions and Sodium Peroxdisul ...
We have to use the data in the table to find the concentration of Iodide ions and Sodium Peroxdisulfate ions


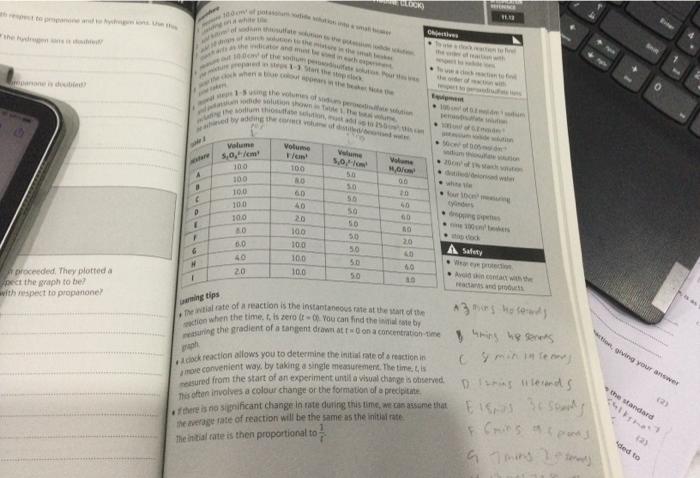
Thas efoen involves a colbur change or the formaton of a predpiate of there is no sifpricant change in tate duireg this lime, we ran asstme that Me artage fale of reciction will be the same as the initial rate the inthial fate is then proportional to \( \frac{1}{f} \).
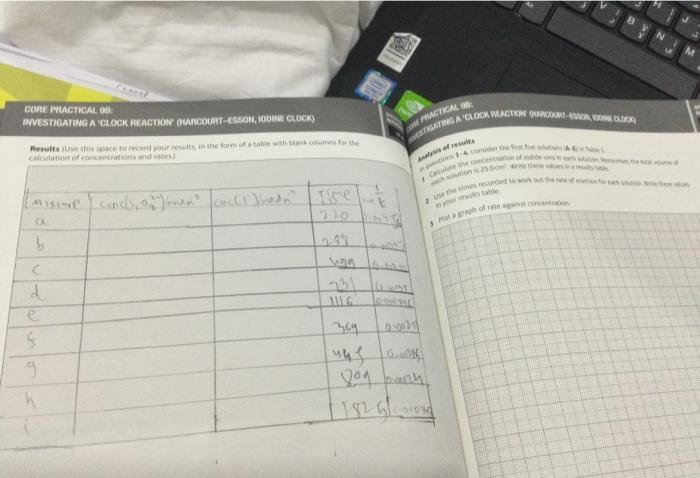
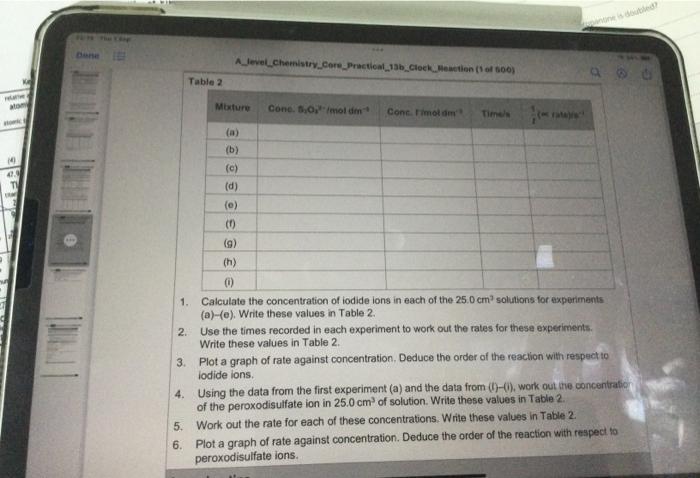
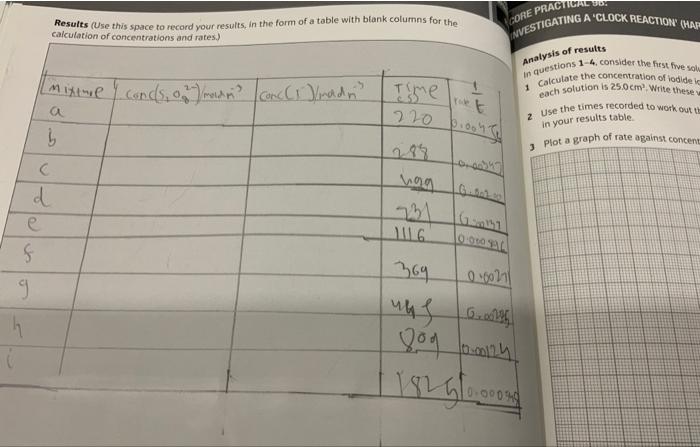
1. Calculate the concentration of iodide ions in each of the \( 25.0 \mathrm{~cm}^{7} \) solutions for eaxpuriments (a)-(e). Write these values in Table 2. 2. Use the times recorded in each experiment to work out the rates for these experiments. Write these values in Table \( 2 . \) 3. Plot a graph of rate against concentration. Deduce the order of the reaction with respect to lodide lons. 4. Using the data from the first experiment (a) and the data from (t)-(i), work out the concentiraici of the peroxodisulfate ion in \( 25.0 \mathrm{~cm}^{3} \) of solution. Write these values in Table \( 2 . \) 5. Work out the rate for each of these concentrations. Write these values in Table 2 . 6. Plot a graph of fate against concentration. Deduce the order of the reaction with respect to peroxodisulfate ions.
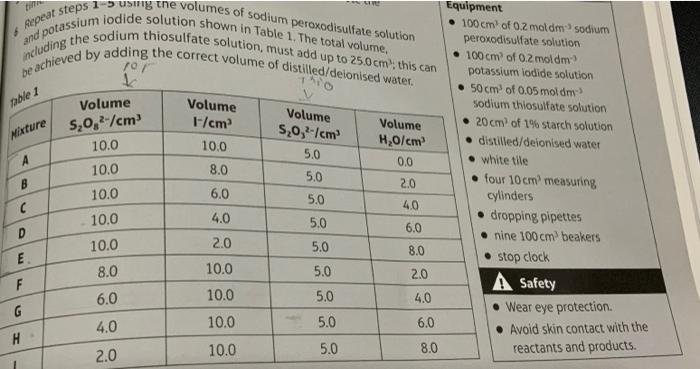
6 aepeat steps is iodide solution shown sodium peroxodisulfate solution and poding the sodium thiosulfate solution, must the total volume, - \( 100 \mathrm{~cm}^{2} \) of \( 0.2 \mathrm{moldm}{ }^{3} \) sodium peroxodisulfate solution \( 50 \mathrm{~cm}^{3} \) of \( 0.05 \) mold dm-3 sodium thiosulfate solution - \( 20 \mathrm{~cm}^{2} \) of \( 1 \% \) starch solution - distilled/deionised water - white tlle - four \( 10 \mathrm{~cm}^{2} \) measuring cylinders - dropping pipettes - nine \( 100 \mathrm{~cm}^{3} \) beakers - stop clock A Safety - Wear eye protection. - Avoid skin contact with the reactants and products.
Results (Use this space to record your results, in the form of a table with btank columns for the WNESTIGATING A 'CLOCK REACTION (HAF calculation of concentrations and rates.) Analysis of results In questions 1-4, consider the first five sol. 1 Calculate the concentration of iodide ic 2 Use the times recorded to work out ti in your results table. - Ptot a graph of rate against concent
Expert Answer
Sample of calculation: the concentrations of I- and S2O82- in each mixture, is obtained using the equation of dilution: M1V1=M2V2. For example, in mixture a: -Calculation of [I-]: M1= 0.2 mol.dm3, V1