Home /
Expert Answers /
Chemistry /
water-vapor-h2o-reacting-with-hot-iron-fe-produces-fe3o4-and-h2-gas-3fe-s-4h2-pa408
(Solved): Water vapor (H2O) reacting with hot iron ( Fe ) produces Fe3O4 and H2 gas: 3Fe(s)+4H2 ...
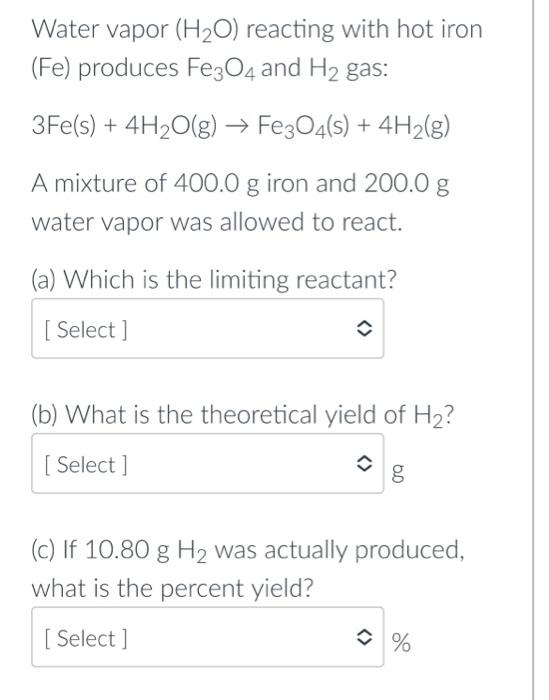
Water vapor reacting with hot iron ( ) produces and gas: A mixture of iron and water vapor was allowed to react. (a) Which is the limiting reactant? (b) What is the theoretical yield of ? (c) If was actually produced, what is the percent yield?
Expert Answer
One mole of each substance contains molar mass of that substanceSo number of moles of a substance in a definite given mass =goven mass÷molar mass of t