Home /
Expert Answers /
Chemistry /
using-the-reagents-below-list-in-order-by-letter-no-period-those-necessary-to-transform-1-chlor-pa785
(Solved): Using the reagents below, list in order (by letter, no period) those necessary to transform 1-chlor ...
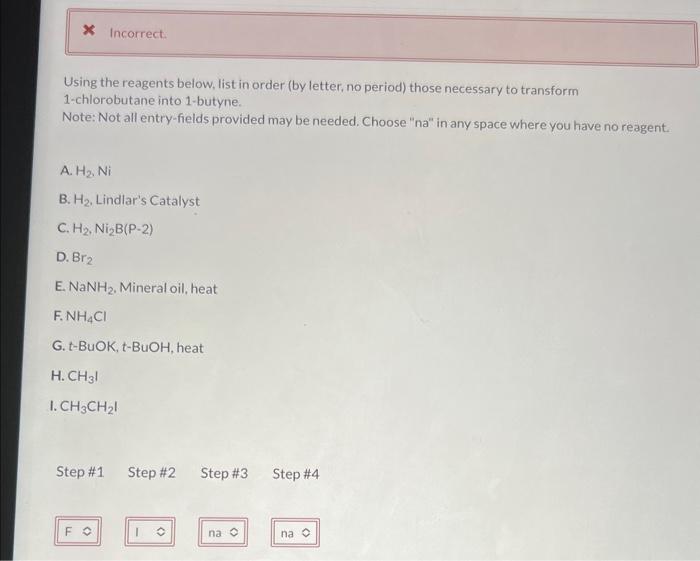
Using the reagents below, list in order (by letter, no period) those necessary to transform 1-chlorobutane into 1-butyne. Note: Not all entry-fields provided may be needed. Choose "na" in any space where you have no reagent. A. \( \mathrm{H}_{2} \mathrm{Ni} \) B. \( \mathrm{H}_{2} \). Lindlar's Catalyst C. \( \mathrm{H}_{2}, \mathrm{Ni}_{2} \mathrm{~B}(\mathrm{P}-2) \) D. \( \mathrm{Br}_{2} \) E. \( \mathrm{NaNH}_{2} \). Mineral oil, heat \( \mathrm{F.} \mathrm{NH}_{4} \mathrm{Cl} \) G. t-BuOK, t-BuOH, heat H. \( \mathrm{CH}_{3} \mathrm{I} \) I. \( \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{I} \)
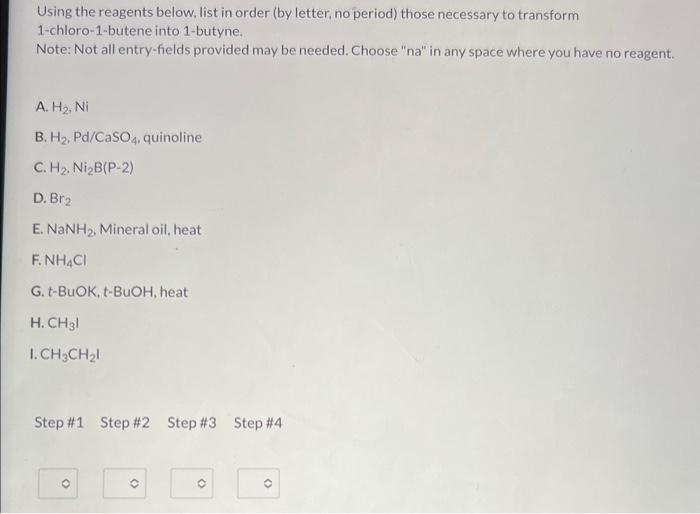
Using the reagents below, list in order (by letter, no period) those necessary to transform 1-chloro-1-butene into 1-butyne. Note: Not all entry-fields provided may be needed. Choose "na" in any space where you have no reagent. A. \( \mathrm{H}_{2}, \mathrm{Ni} \) B. \( \mathrm{H}_{2}, \mathrm{Pd} / \mathrm{CaSO}_{4} \), quinoline C. \( \mathrm{H}_{2}, \mathrm{Ni} \mathrm{i}_{2} \mathrm{~B}(\mathrm{P}-2) \) D. \( \mathrm{Br}_{2} \) E. \( \mathrm{NaNH}_{2} \). Mineral oil, heat \( \mathrm{F} . \mathrm{NH}_{4} \mathrm{Cl} \) G.t-BuOK, t-BuOH, heat H. \( \mathrm{CH}_{3} \mathrm{l} \) 1. \( \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{I} \) Step \#1 Step \#2
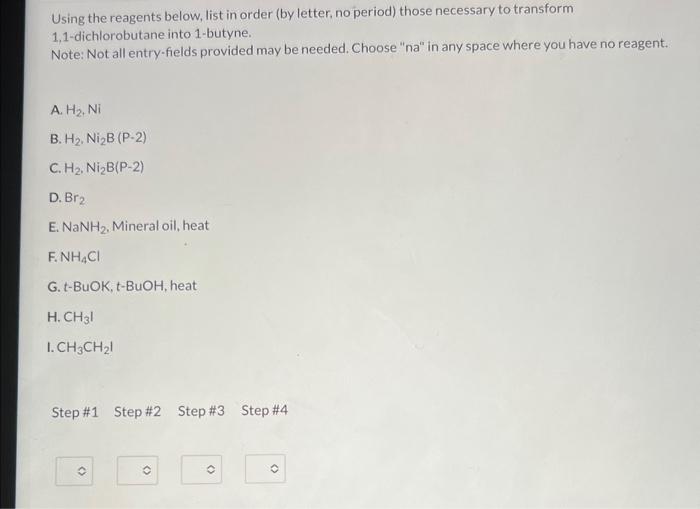
Using the reagents below, list in order (by letter, no period) those necessary to transform 1,1-dichlorobutane into 1-butyne. Note: Not all entry-fields provided may be needed. Choose "na" in any space where you have no reagent. A. \( \mathrm{H}_{2}, \mathrm{Ni} \) B. \( \mathrm{H}_{2}, \mathrm{Ni}_{2} \mathrm{~B}(\mathrm{P}-2) \) C. \( \mathrm{H}_{2}, \mathrm{Ni} \mathrm{i}_{2} \mathrm{~B}(\mathrm{P}-2) \) D. \( \mathrm{Br}_{2} \) E. \( \mathrm{NaNH}_{2} \). Mineral oil, heat F. \( \mathrm{NH}_{4} \mathrm{Cl} \) G. t-BuOK, t-BuOH, heat H. \( \mathrm{CH}_{3} \mathrm{I} \) I. \( \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{I} \) Step \#1 Step \#2 Step\#3