Home /
Expert Answers /
Chemistry /
using-the-provided-table-and-the-equation-below-determine-the-heat-of-formation-in-mathrm-kj-pa612
(Solved): Using the provided table and the equation below, determine the heat of formation (in \( \mathrm{kJ} ...
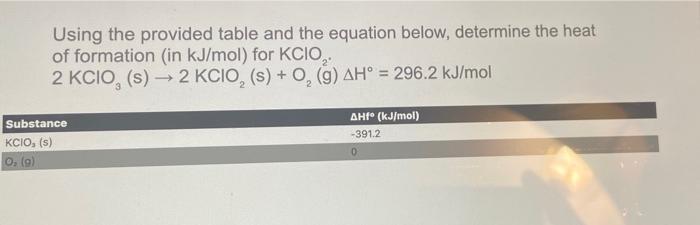
Using the provided table and the equation below, determine the heat of formation (in \( \mathrm{kJ} / \mathrm{mol} \) ) for \( \mathrm{KClO}_{2} \). \[ 2 \mathrm{KClO}_{3}(\mathrm{~s}) \rightarrow 2 \mathrm{KClO}_{2}(\mathrm{~s})+\mathrm{O}_{2}(\mathrm{~g}) \Delta \mathrm{H}^{\circ}=296.2 \mathrm{~kJ} / \mathrm{mol} \]