Home /
Expert Answers /
Chemistry /
using-the-half-reaction-method-balance-the-following-redox-equation-in-an-acidic-aqueous-solution-pa709
(Solved): Using the Half-Reaction method, balance the following Redox equation in an acidic aqueous solution: ...
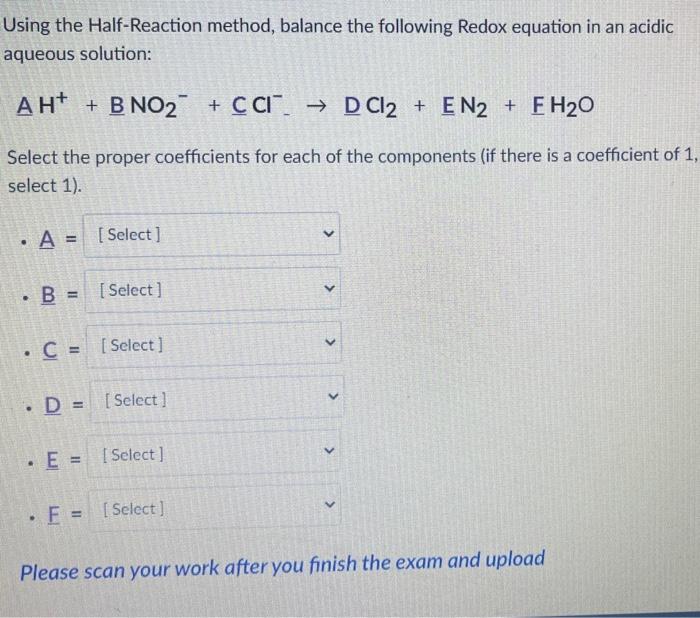
Using the Half-Reaction method, balance the following Redox equation in an acidic aqueous solution: \[ \mathrm{AH}^{+}+\mathrm{BNO}_{2}^{-}+\mathrm{CCl}^{-} \rightarrow \mathrm{DCl}_{2}+\mathrm{EN}_{2}+\mathrm{EH}_{2} \mathrm{O} \] Select the proper coefficients for each of the components (if there is a coefficient of 1 , select 1). - \( \mathrm{A}= \) - \( \underline{B}= \) - \( \underline{C}= \) - \( \mathrm{D}= \) - \( \underline{E}= \) - \( E= \) Please scan your work after you finish the exam and upload