Home /
Expert Answers /
Chemistry /
using-the-following-information-compute-density-mass-volume-to-the-correct-number-of-significa-pa610
(Solved): Using the following information, compute density (= mass/volume) to the correct number of significa ...
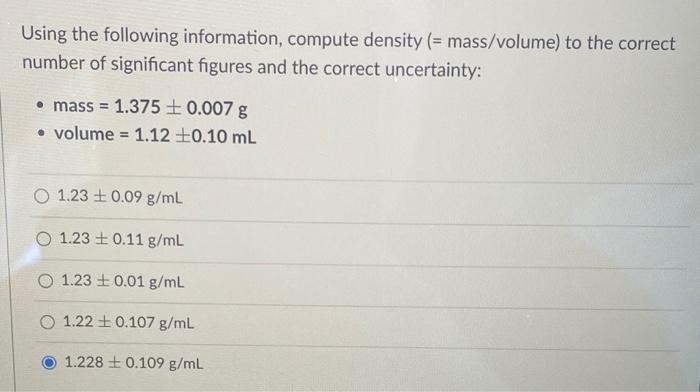
Using the following information, compute density (= mass/volume) to the correct number of significant figures and the correct uncertainty: - mass \( =1.375 \pm 0.007 \mathrm{~g} \) - volume \( =1.12 \pm 0.10 \mathrm{~mL} \) \( 1.23 \pm 0.09 \mathrm{~g} / \mathrm{mL} \) \( 1.23 \pm 0.11 \mathrm{~g} / \mathrm{mL} \) \( 1.23 \pm 0.01 \mathrm{~g} / \mathrm{mL} \) \( 1.22 \pm 0.107 \mathrm{~g} / \mathrm{mL} \) \( 1.228 \pm 0.109 \mathrm{~g} / \mathrm{mL} \)
Expert Answer
In the following question, we always calculate uncertainty separately. We know that, So, But fo