Home /
Expert Answers /
Chemistry /
using-the-bond-energies-provided-calculate-the-enthalpy-of-the-reaction-triangle-mathrm-hrx-pa596
(Solved): Using the bond energies provided, calculate the enthalpy of the reaction ( \( \triangle \mathrm{Hrx ...
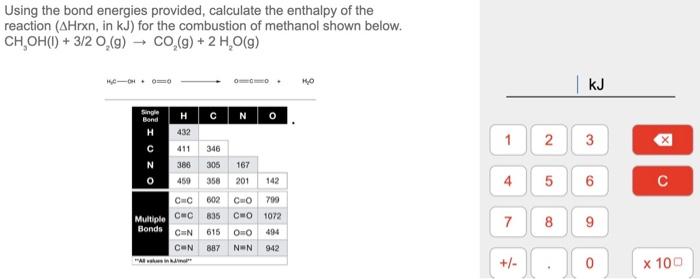
Using the bond energies provided, calculate the enthalpy of the reaction ( \( \triangle \mathrm{Hrxn} \), in \( \mathrm{kJ} \) ) for the combustion of methanol shown below. \( \mathrm{CH}_{3} \mathrm{OH}(\mathrm{l})+3 / 2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \)
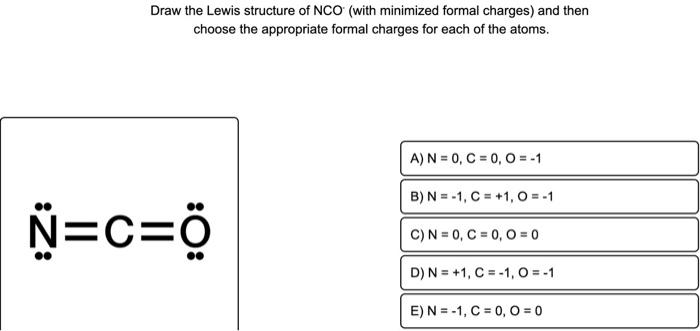
Draw the Lewis structure of NCO (with minimized formal charges) and then choose the appropriate formal charges for each of the atoms. D) \( \mathrm{N}=+1, \mathrm{C}=-1, \mathrm{O}=-1 \)
Expert Answer
CH3OH(l) + 3/2O2(g) -----------> CO2(g) + 2H2O(g) DH = bond energies of reactans - bond energies