Home /
Expert Answers /
Chemistry /
using-the-biochemical-standard-reduction-potentials-listed-in-table-14-1-calculate-the-change-in-st-pa250
(Solved): Using the biochemical standard reduction potentials listed in Table 14.1, calculate the change in st ...
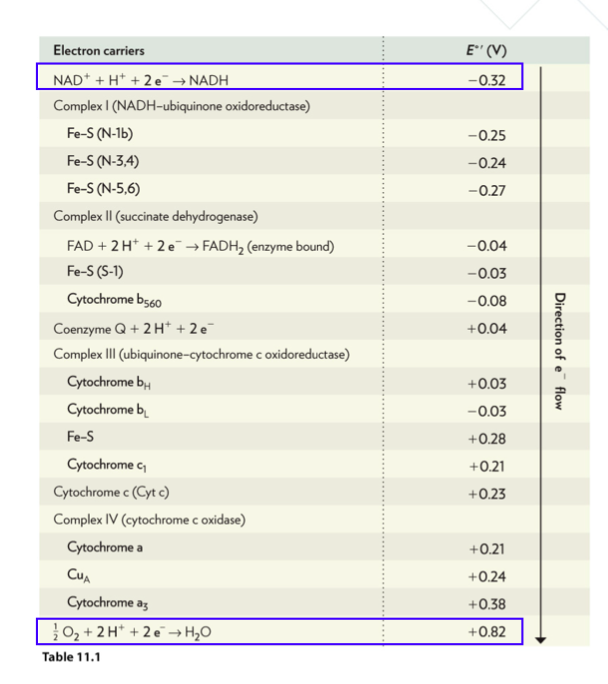
Using the biochemical standard reduction potentials listed in Table 14.1, calculate the change in standard Gibbs energy when FADH2 is oxidized by Complex II.
\table[[Electron carriers,E^('')(V)],[NAD^(+)+H^(+)+2e^(-)->NADH,-0.32],[Complex I (NADH-ubiquinone oxidoreductase),],[Fe-S(N-1b),-0.25],[Fe-S(N-34),-0.24],[Fe-S(N-56),-0.27],[Complex II (succinate dehydrogenase),],[FAD+2H^(+)+2e^(-)->FADH_(2) (enzyme bound),-0.04],[Fe-S(S-1),-0.03],[Cytochrome b_(560),-0.08],[Coenzyme C+2H^(+)+2e^(-),+0.04],[Complex III (ubiquinone-cytochrome c oxidoreductase),],[Cytochrome b H_(H),+0.03],[Cytochrome b b_(L),-0.03],[Fe -S,+0.28],[Cytochrome C_(1),+0.21],[Cytochrome c(Cytc),+0.23],[Complex IV (cytochrome c oxidase),],[Cytochrome a,+0.21],[Cu_(A),+0.24],[Cytochrome a3,+0.38],[(1)/(2)O_(2)+2H^(+)+2e^(-)->H_(2)O,+0.82]]
Table 11.1