Home /
Expert Answers /
Chemical Engineering /
using-kopp-39-s-rule-estimate-the-heat-capacity-of-2-methylheptane-at-20-c-at-20-c-2-methylhep-pa132
(Solved): Using Kopp's rule, estimate the heat capacity of 2-methylheptane at 20 C. At 20 C, 2-methylhep ...
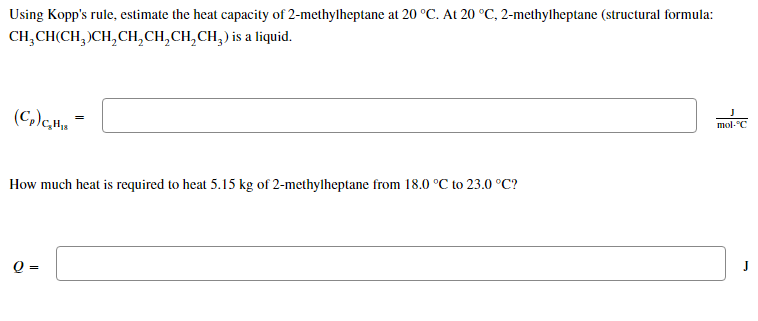
Using Kopp's rule, estimate the heat capacity of 2-methylheptane at 20 °C. At 20 °C, 2-methylheptane (structural formula: CH?CH(CH?)CH?CH?CH?CH?CH?) is a liquid. J (C,)cH = mol-C How much heat is required to heat 5.15 kg of 2-methylheptane from 18.0 °C to 23.0 °C? Q = J
Expert Answer
Answer: Kopp found "that the molecular heat capacity of a solid compound is the sum of the atomic heat capacities of the elements composing it; the eleme