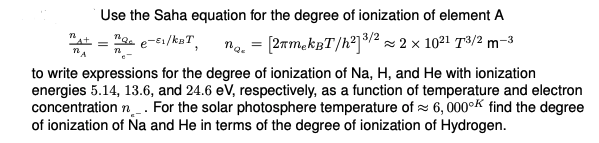Home /
Expert Answers /
Advanced Physics /
use-the-saha-equation-for-the-degree-of-ionization-of-element-a-frac-n-delta-n-a-pa577
(Solved): Use the Saha equation for the degree of ionization of element \( A \) \[ \frac{n_{\Delta}+}{n_{A}} ...
Use the Saha equation for the degree of ionization of element \( A \) \[ \frac{n_{\Delta}+}{n_{A}}=\frac{n_{Q_{e}}}{n_{e^{-}}} e^{-\varepsilon_{1} / k_{B} T}, \quad n_{Q_{\varepsilon}}=\left[2 \pi m_{e} k_{B} T / h^{2}\right]^{3 / 2} \approx 2 \times 10^{21} T^{3 / 2} \mathrm{~m}^{-3} \] to write expressions for the degree of ionization of \( \mathrm{Na}, \mathrm{H} \), and \( \mathrm{He} \) with ionization energies \( 5.14,13.6 \), and \( 24.6 \mathrm{eV} \), respectively, as a function of temperature and electron concentration \( n_{\varepsilon_{-}} \). For the solar photosphere temperature of \( \approx 6,000^{\circ K} \) find the degree of ionization of \( \mathrm{Na} \) and \( \mathrm{He} \) in terms of the degree of ionization of Hydrogen.