Home /
Expert Answers /
Mechanical Engineering /
use-the-phase-diagram-below-a-copper-nickel-alloy-53-wt-ni-and-47-wt-cu-is-1-heated-to-1400c-pa694
(Solved): Use the phase diagram below. A copper-nickel alloy (53 wt% Ni and 47 wt% Cu) is 1) heated to 1400C ...
Use the phase diagram below. A copper-nickel alloy (53 wt% Ni and 47 wt% Cu) is 1) heated to 1400°C, and then 2) rapidly cooled to 1300 °C and keep at this temperature for long enough time to reach to an equilibrium condition; and then 3) the temperature is quenched to room temperature.
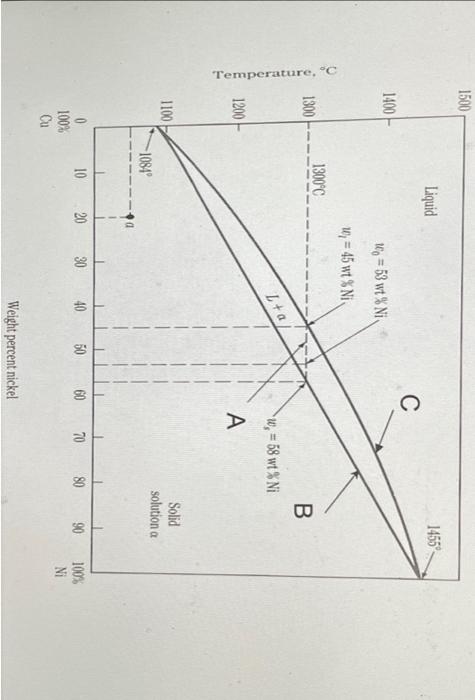

What is the phase/s after process 2) ?


Temperature, °C 1500 1400 1300 1200 1100 100% Cu 1300°C -1084? Liquid 10 153 wt% Niv w = 45 wt% Ni 20 30 L+a 40 C 50 60 Weight percent nickel w, = 58 wt% Ni A B 1455- Solid solution a 70 80 90 100% Ni
O A. a-solid + a'solid (a', the same structure but different composition with a) O B. Liquid + a-solid OC. a-solid OD. Liquid
Expert Answer
Please find your answer in