Home /
Expert Answers /
Chemistry /
use-the-nernst-equation-to-calculate-the-cell-potentials-in-v-of-the-following-cells-at-298-15-k-pa307
(Solved): Use the Nernst equation to calculate the cell potentials (in V) of the following cells at 298.15 K. ...
Use the Nernst equation to calculate the cell potentials (in V) of the following cells at 298.15 K. 2+ (a) 2 Ag (ag, 0.20 M) + Co(s) ? 2 Ag(s) + Co V 2- (b) Pb(s) + SO4 (aq, 0.30 M) + 2 AgBr(s) ? PbSO4(s) + 2 Ag(s) + 2 Br (aq, 0.20 M) V (aq, 0.65 M) 2- 2+ (c) Co(s) + PtCl (aq, 0.10 M)? Co (aq, 0.60 M) + PtCl(aq, 0.10 M) + 2 C1 (aq, 0.40 M) V 2-
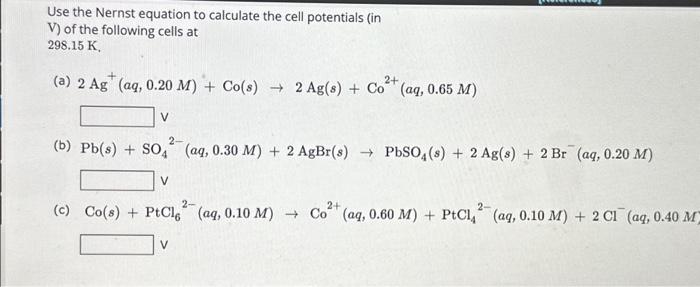
Use the Nernst equation to calculate the cell potentials (in V) of the following cells at (a) (b) (c)
Expert Answer
The Nernst equation relates the cell potential of an electrochemical cell to the standard electrode potential and the activities (or concentrations) of the species involved:where E is the cell potential, E° is the standard electrode potential, R is the gas constant, T is the temperature in Kelvin, n is the number of electrons transferred in the reaction, F is the Faraday constant, and Q is the reaction quotient.Using this equation, we can calculate the cell potentials of the given cells at 298.15 K as follows:(a)The half-reactions involved are:The overall reaction is the sum of the two half-reactions:The reaction quotient Q is:At equilibrium, the cell potential E is equal to zero, so we can solve for E°:Substituting the given values, we get:E° = (8.314 J/K/mol) * (298.15 K) / (2 * 96485 C/mol) * ln (0.20 M / 0.65 M)^2 E° = -0.068 VThus, the cell potential is:E = -0.068 V - (RT/2F) ln [Co2+]/[Ag+]^2Substituting the given values, we get:E = -0.068 V - (8.314 J/K/mol * 298.15 K / (2 * 96485 C/mol)) * ln (0.65 M / 0.20 M)^2 E = -0.068 V - 0.127 V E = -0.195 VTherefore, the cell potential of the given cell is -0.195 V.