Home /
Expert Answers /
Chemistry /
use-the-conservation-of-mass-interactive-to-answer-the-question-consider-the-reaction-mathrm-pa275
(Solved): Use the Conservation of Mass Interactive to answer the question. Consider the reaction. \[ \mathrm{ ...
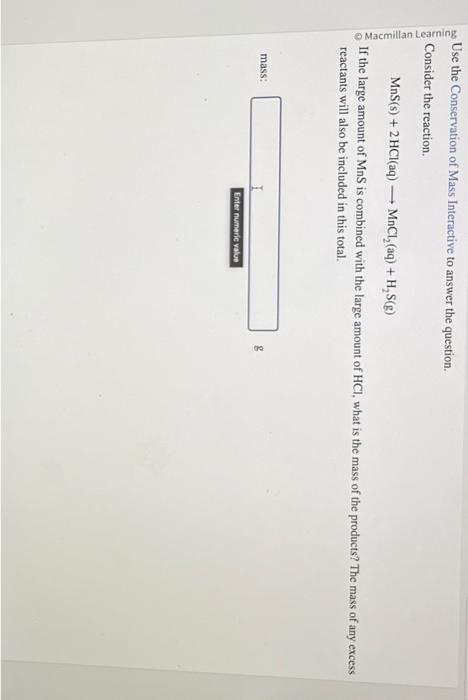
Use the Conservation of Mass Interactive to answer the question. Consider the reaction. \[ \mathrm{MnS}(\mathrm{s})+2 \mathrm{HCl}(\mathrm{aq}) \longrightarrow \mathrm{MnCl}_{2}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{~S}(\mathrm{~g}) \] If the large amount of \( \mathrm{MnS} \) is combined with the large amount of \( \mathrm{HCl} \), what is the mass of the products? The mass of any excess reactants will also be included in this total. mass: g
Expert Answer
Excellent question for understanding the stoichiometric calculation. In this reaction one mole of MnS needs two mole of HCl to react completely..and one mol MnCl2 and one mole H2S will form. But we have to in mind the the number of moles is related t