Home /
Expert Answers /
Chemistry /
use-the-balanced-equation-for-the-combustion-of-ethane-to-complete-the-table-2ch6-g-70-g-pa890
(Solved): Use the balanced equation for the combustion of ethane to complete the table. 2CH6(g) + 70(g ...
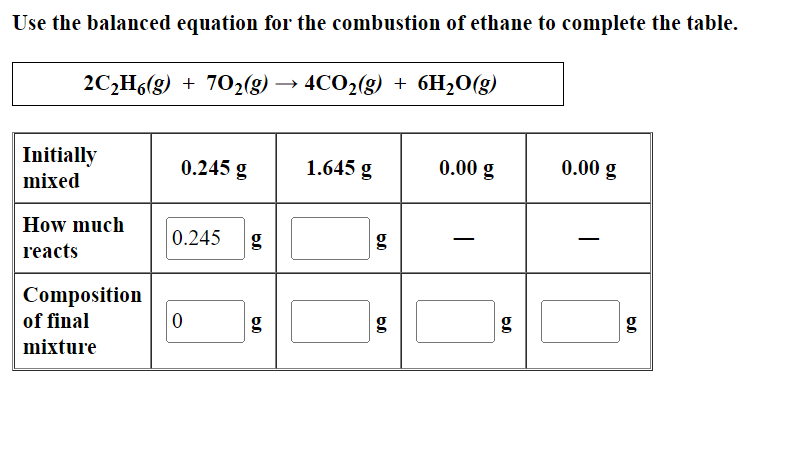
Use the balanced equation for the combustion of ethane to complete the table. 2C?H6(g) + 70?(g) > 4CO?(g) + 6H?O(g) Initially mixed How much reacts Composition of final mixture 0.245 g 0.245 g 0 g 1.645 g g 6.0 0.00 g g 0.00 g 6.0