Home /
Expert Answers /
Chemistry /
use-standard-enthalpies-of-formation-to-calculate-hrxn-for-the-following-reaction-c2h5-pa472
(Solved): Use standard enthalpies of formation to calculate Hrxn for the following reaction: C2H5 ...
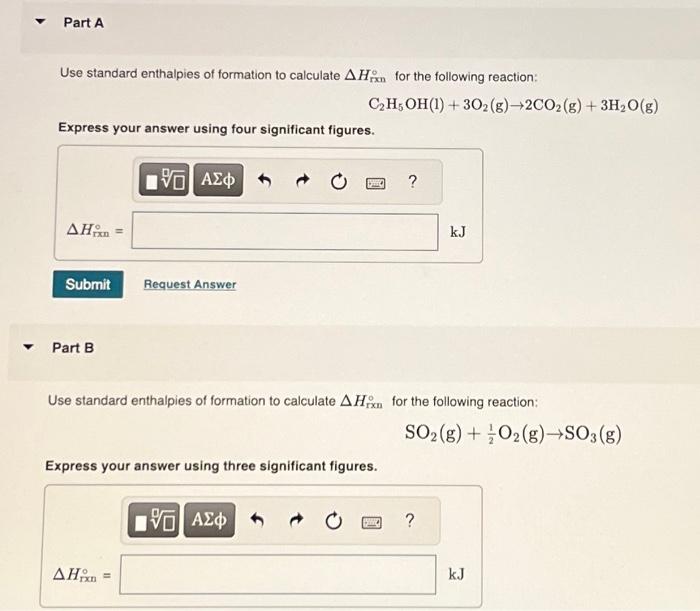
Use standard enthalpies of formation to calculate for the following reaction: Express your answer using four significant figures. Part B Use standard enthalpies of formation to calculate for the following reaction: Express your answer using three significant figures.
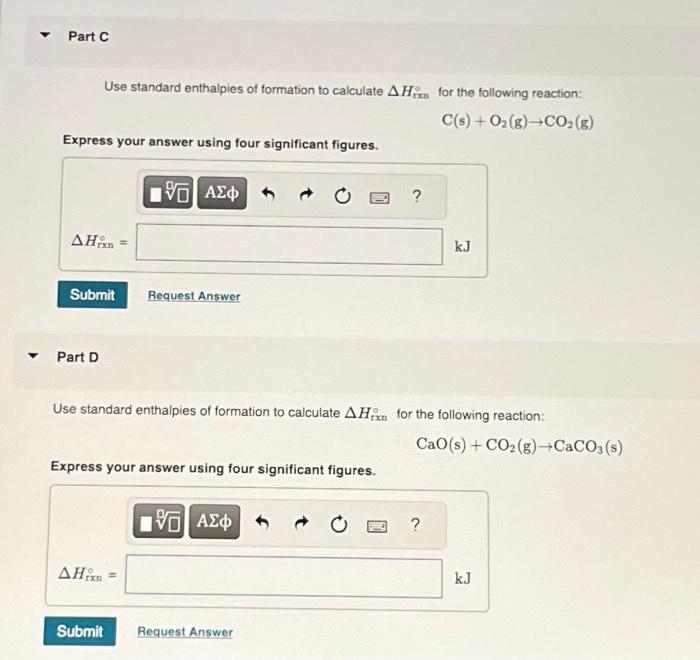
Use standard enthalpies of formation to calculate for the following reaction: Express your answer using four significant figures. Part D Use standard enthalpies of formation to calculate for the following reaction: Express your answer using four significant figures.