Home /
Expert Answers /
Chemistry /
use-ka-of-acetic-acid-and-h-in-the-initial-solution-to-calculate-the-concentrat-pa962
(Solved): Use Ka of acetic acid and H+ in the initial solution to calculate the concentrat ...

Use Ka of acetic acid and H+ in the initial solution to calculate the concentration M of the acetic acid unknown #2 solution.
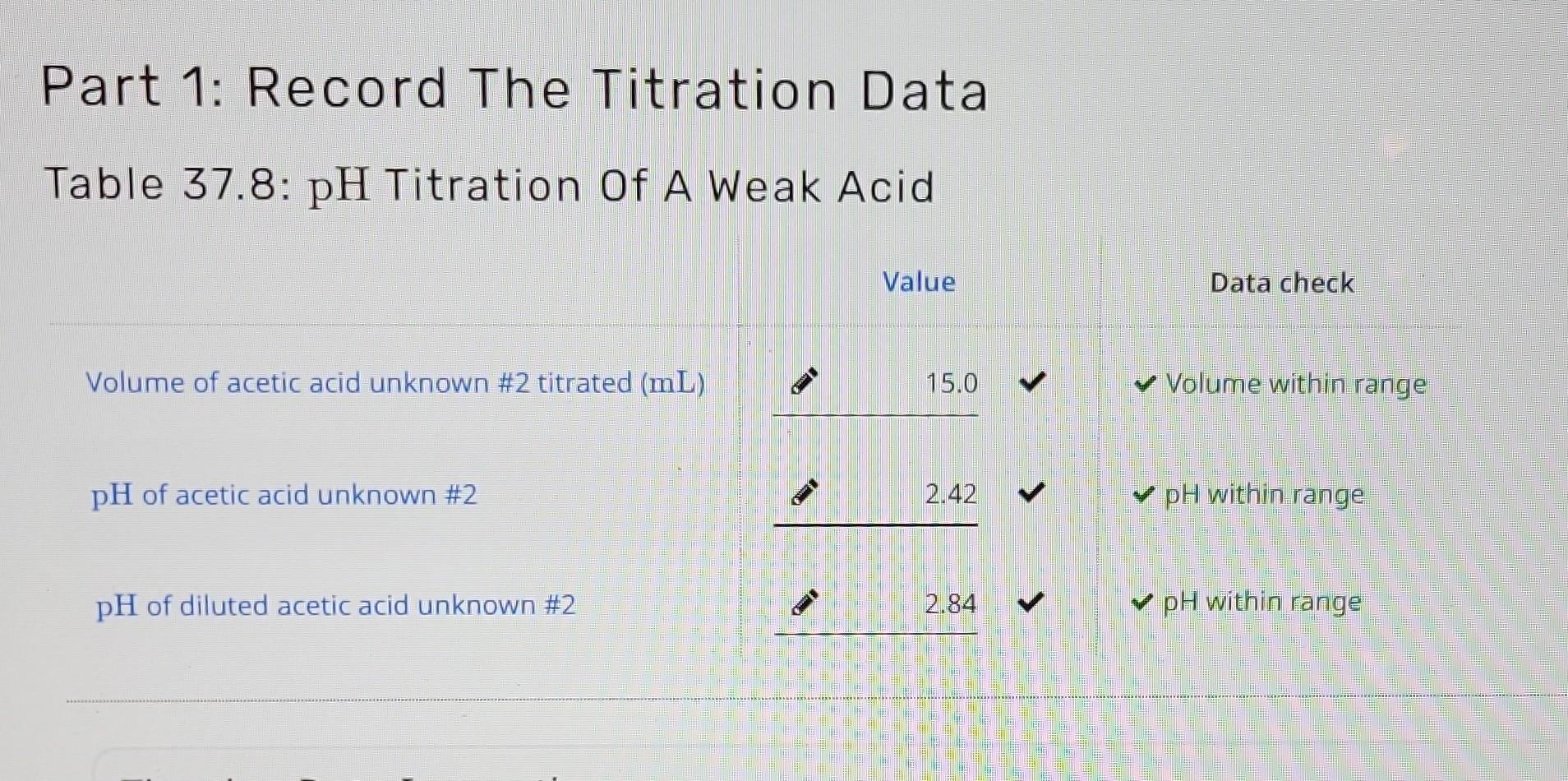
Part 1: Record The Titration Data Table 37.8: pH Titration Of A Weak Acid
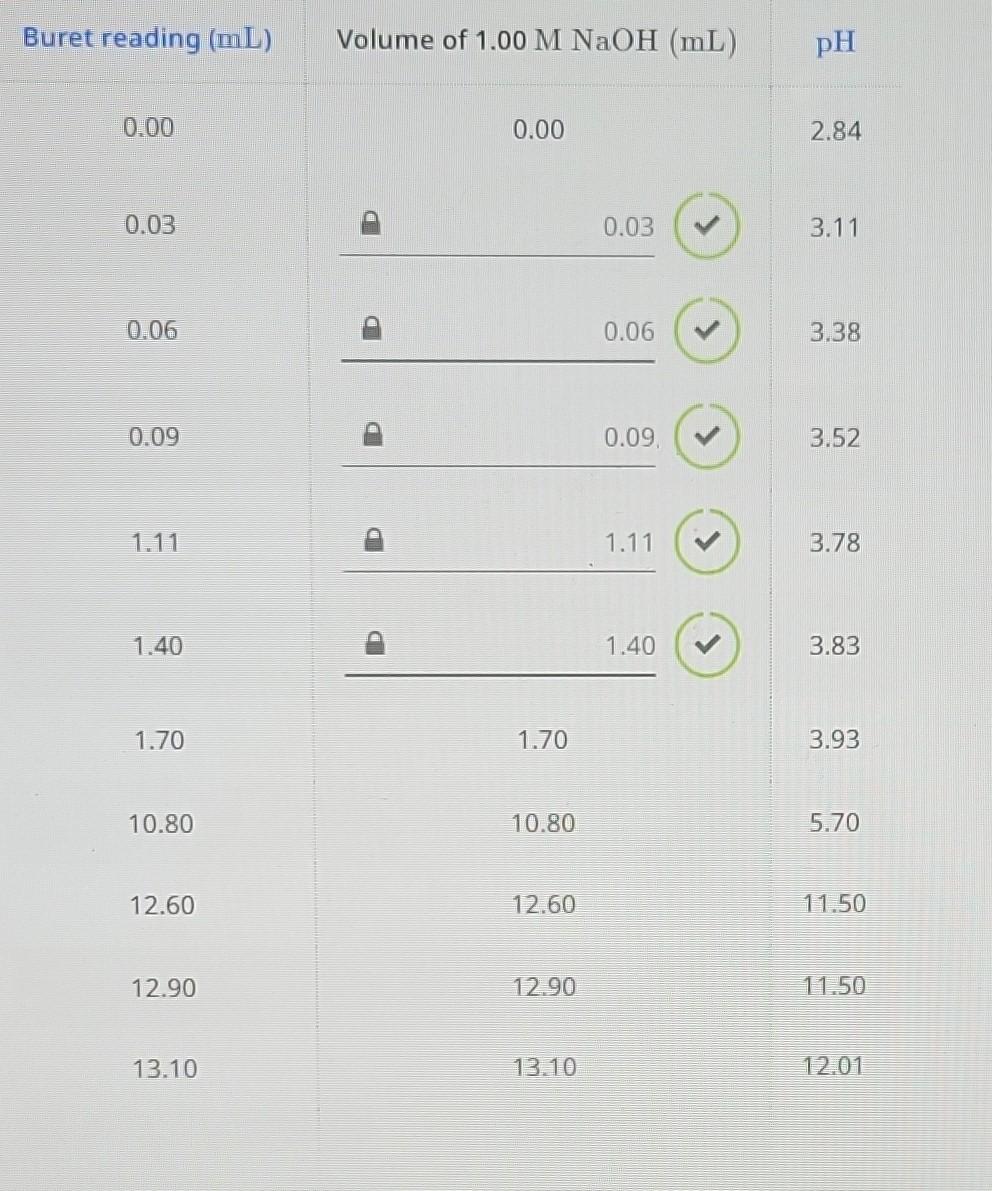
Buret reading (mL) Volume of 0.00 0.00 2.84 0.03 3.11 0.06 ? 0.06 3.38 0.09 ( 3.52 1.11 1.11 3.78 1.40 3.83 1.70 1.70 3.93 10.80 10.80 5.70 12.60 12.60 11.50 12.90 12.90 11.50 13.10 13.10 12.01
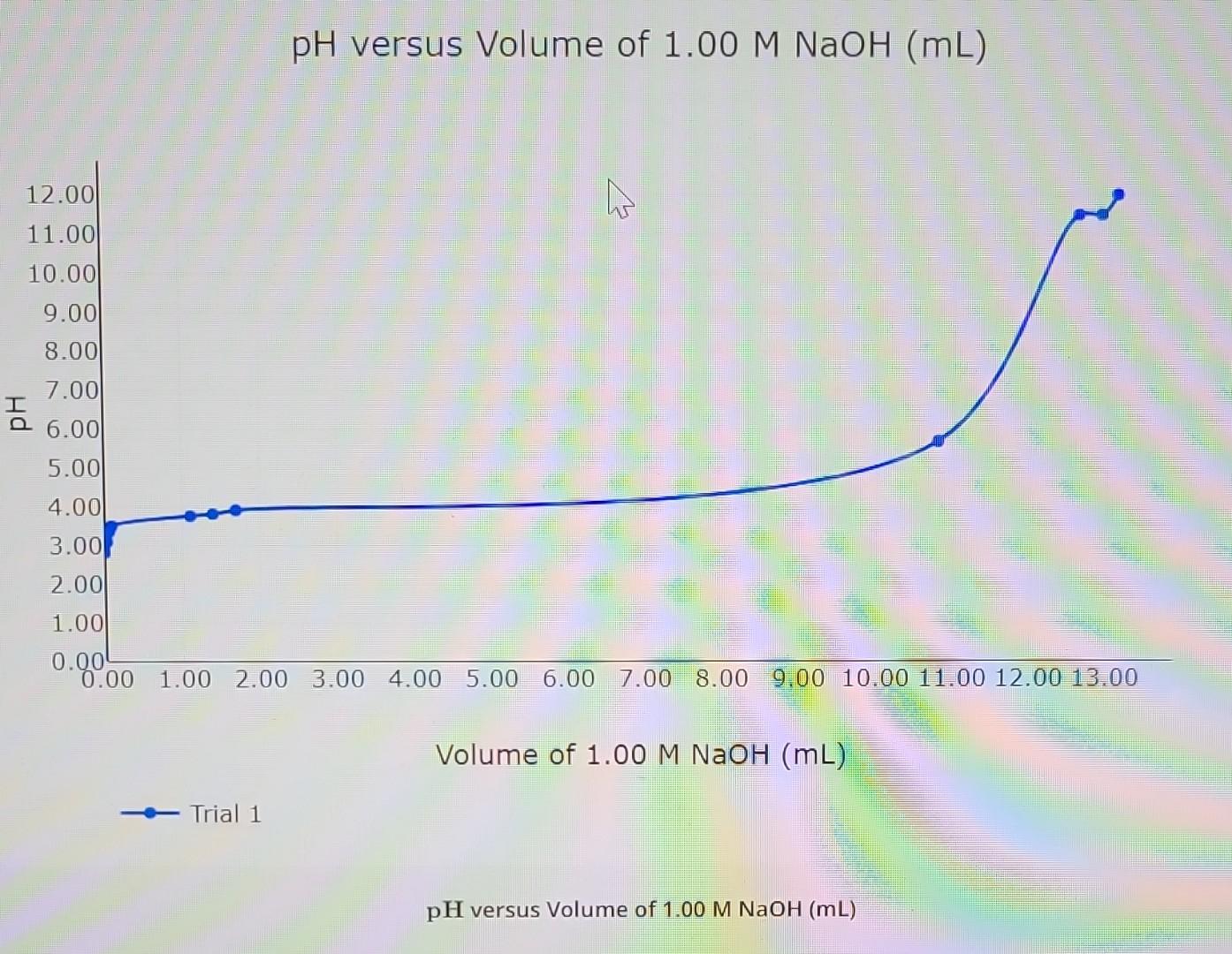
versus Volume of versus Volume of
Value What is the of the solution at the end point of the titratiop? What is the volume of solution dispensed at the end point? Use the titration curve to determine the of acetic acid. What is the for acetic acid? Calculate in the acetic acid unknown \#2 solution based on the of the solution before it was diluted.
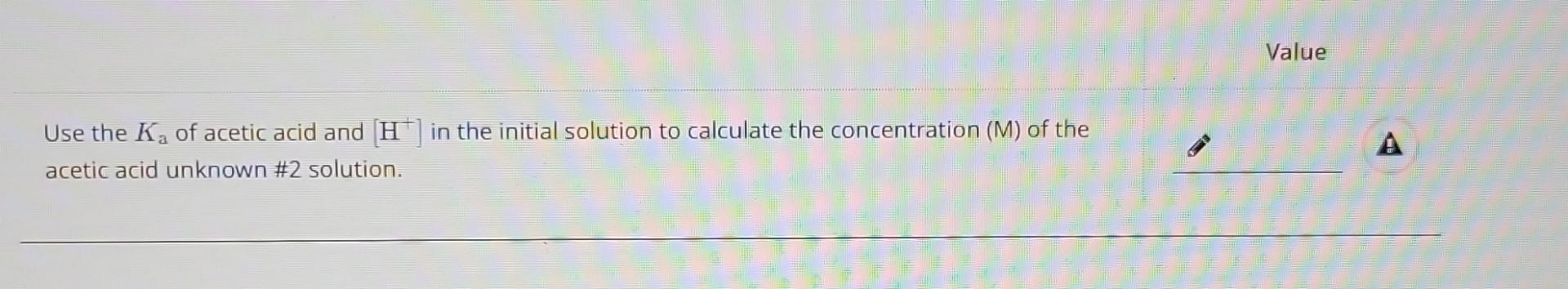
Use the of acetic acid and in the initial solution to calculate the concentration (M) of the acetic acid unknown \#2 solution.