Home /
Expert Answers /
Chemistry /
use-constant-pressure-calorimetry-to-determine-enthalpy-change-the-salt-cesium-perchlorate-is-sol-pa691
(Solved): Use constant-pressure calorimetry to determine enthalpy change. The salt cesium perchlorate is sol ...
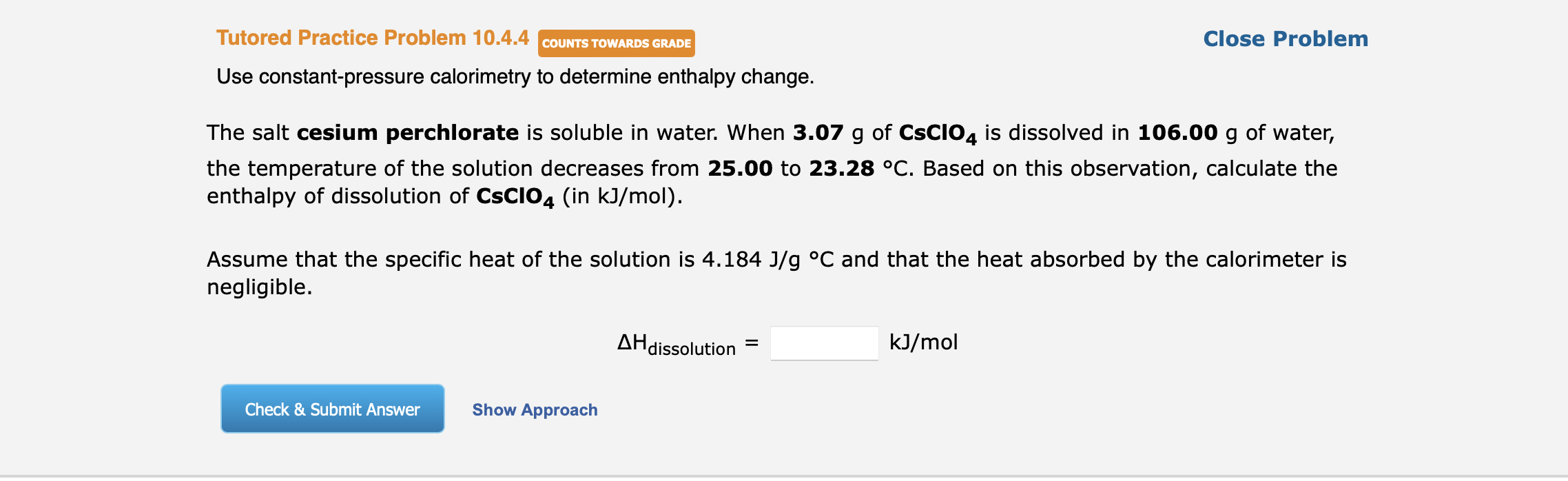
Use constant-pressure calorimetry to determine enthalpy change. The salt cesium perchlorate is soluble in water. When \( 3.07 \mathrm{~g} \) of \( \mathbf{C s C l O}_{4} \) is dissolved in \( 106.00 \mathrm{~g} \) of water, :he temperature of the solution decreases from 25.00 to \( 23.28^{\circ} \mathrm{C} \). Based on this observation, calculate the enthalpy of dissolution of \( \mathbf{C s C l O}_{4} \) (in \( \mathrm{kJ} / \mathrm{mol} \) ). Assume that the specific heat of the solution is \( 4.184 \mathrm{~J} / \mathrm{g}{ }^{\circ} \mathrm{C} \) and that the heat absorbed by the calorimeter is hegligible. \[ \Delta \mathrm{H}_{\text {dissolution }}=\quad \mathrm{kJ} / \mathrm{mol} \]