Home /
Expert Answers /
Computer Science /
use-combustion-analysis-to-determine-empirical-and-molecular-formulas-hydrocarbons-nwhen-5-428-g-pa256
(Solved): Use combustion analysis to determine empirical and molecular formulas (hydrocarbons).\\nWhen 5.428 g ...
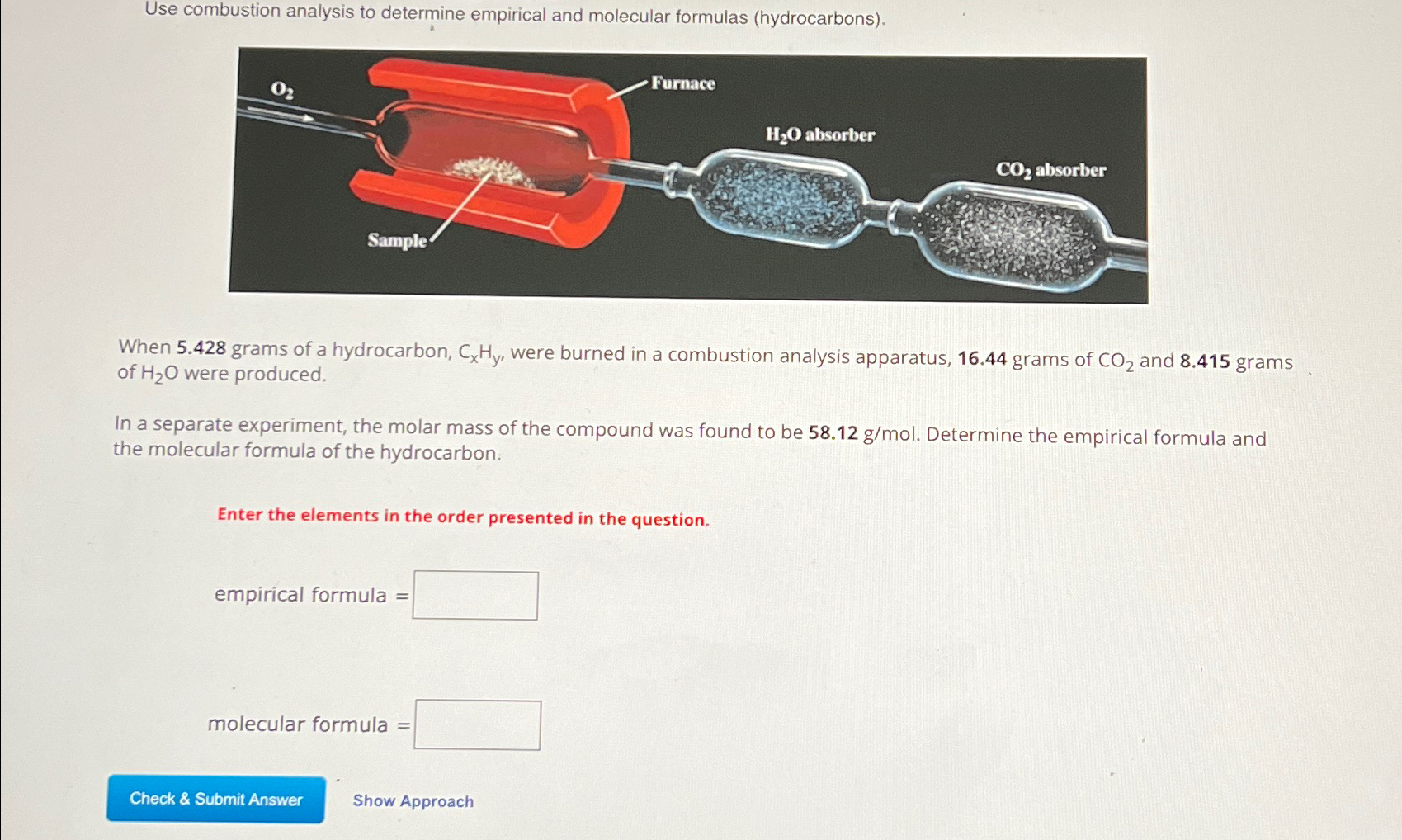
Use combustion analysis to determine empirical and molecular formulas (hydrocarbons).\\nWhen
5.428grams of a hydrocarbon,
C_(x)H_(y), were burned in a combustion analysis apparatus, 16.44 grams of
CO_(2)and 8.415 grams of
H_(2)Owere produced.\\nIn a separate experiment, the molar mass of the compound was found to be
58.12(g)/(m)ol. Determine the empirical formula and the molecular formula of the hydrocarbon.\\nEnter the elements in the order presented in the question.\\nempirical formula
=\\nmolecular formula
=\\nShow Approach