Home /
Expert Answers /
Chemistry /
use-average-bond-enthalpies-given-in-the-table-below-to-calculate-the-enthalpy-change-for-the-fol-pa364
(Solved): Use average bond enthalpies (given in the table below) to calculate the enthalpy change for the fol ...
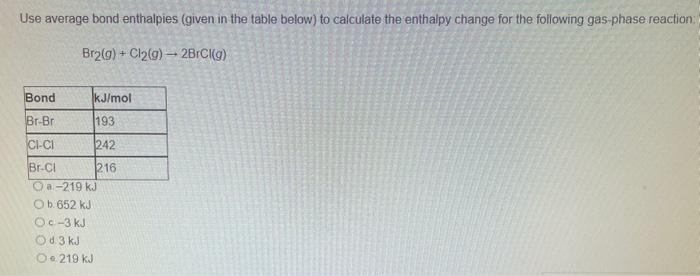
Use average bond enthalpies (given in the table below) to calculate the enthalpy change for the following gas phase reaction: \[ \mathrm{Br}_{2}(g)+\mathrm{Cl}_{2}(g) \rightarrow 2 \mathrm{BrCl}(g) \] b. \( 652 \mathrm{~kJ} \) c \( -3 \mathrm{~kJ} \) d \( 3 \mathrm{~kJ} \) e. \( 219 \mathrm{~kJ} \)