Home /
Expert Answers /
Advanced Physics /
understanding-the-radial-equations-of-hydrogen-atom-radial-equation-of-hydrogen-atom-u-r-pa952
(Solved): Understanding the Radial Equations of Hydrogen Atom Radial equation of Hydrogen atom: U(r) = ...
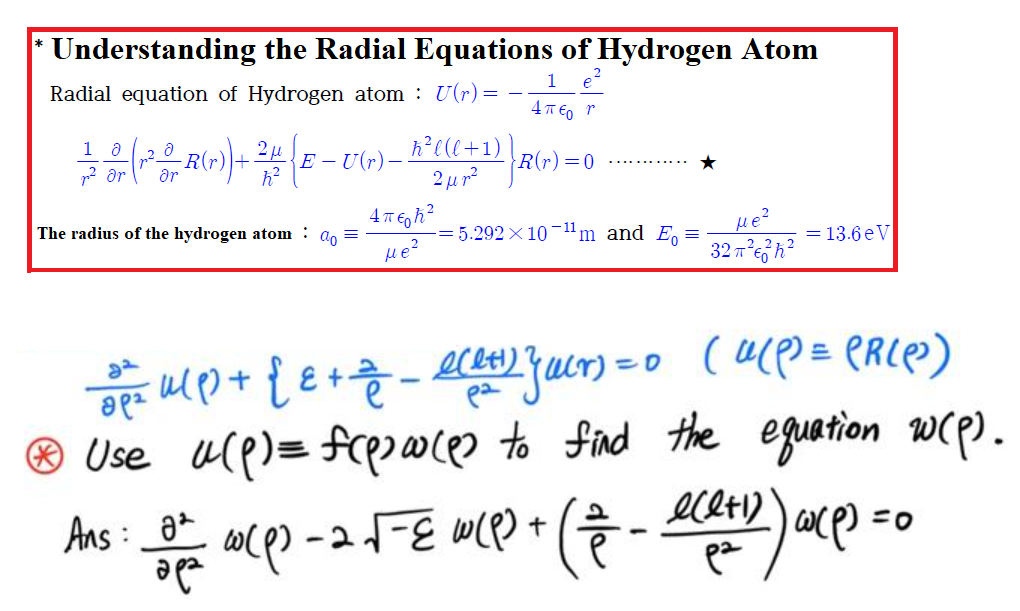
· Understanding the Radial Equations of Hydrogen Atom Radial equation of Hydrogen atom: U(r) = · 1 4? E o r 1 2 0 - (-² ° F (r)) + ²4 (E-U(r) _ A³²(((+1) 2 ?. l (l ar ?r h² R(r) = 0 2?p² The radius of the hydrogen atom ao = 4?6h² -=5.292×10-¹¹m and E? = Me² = 13.6 eV ??? 32?²²h² ??? = u( ² ) + { ? + = _ ece+¹) } acr) = 0 (U(P) = (RIP) (l+1) pa * Use u(p) = f(pouces to find the equation w(p). Ans: Of a(Q) - 2 ?-E W(1) + (7 - ece+D)) axp) = 0 ??? ??