Home /
Expert Answers /
Chemistry /
under-acidic-conditions-c2h4o-is-used-to-titrate-the-dichromate-ion-cr2o7-2-the-dichromate-ion-is-pa163
(Solved): Under acidic conditions C2H4O is used to titrate the dichromate ion, Cr2O7 2-. The dichromate ion is ...
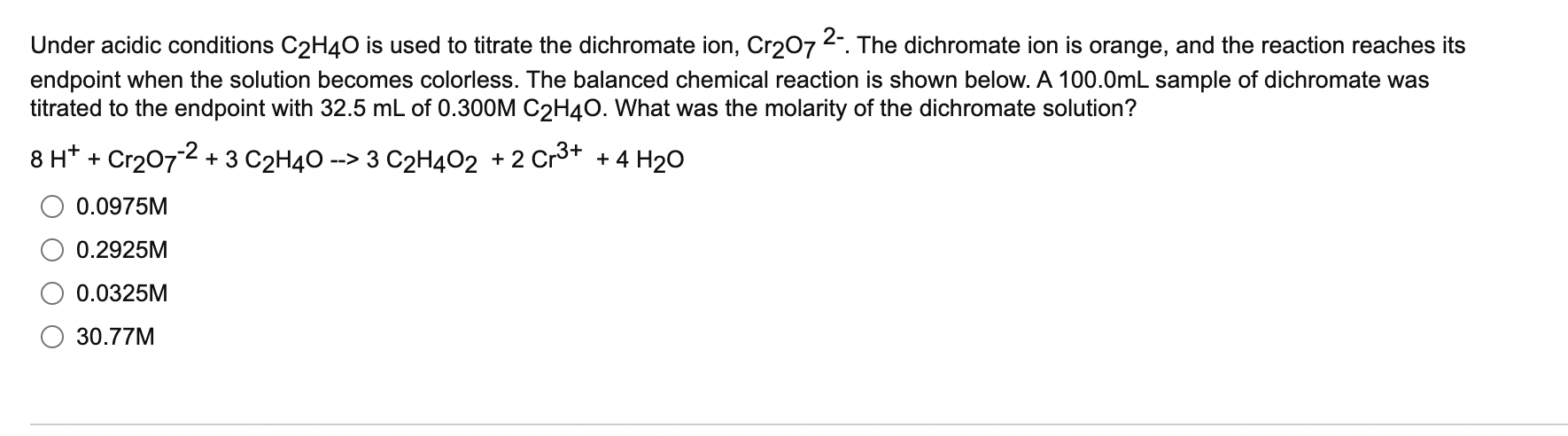
Under acidic conditions \( \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O} \) is used to titrate the dichromate ion, \( \mathrm{Cr}_{2} \mathrm{O}_{7}{ }^{2-} \). The dichromate ion is orange, and the reaction reaches its endpoint when the solution becomes colorless. The balanced chemical reaction is shown below. \( A 100.0 \mathrm{~mL} \) sample of dichromate was titrated to the endpoint with \( 32.5 \mathrm{~mL} \) of \( 0.300 \mathrm{M} \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O} \). What was the molarity of the dichromate solution? \( 8 \mathrm{H}^{+}+\mathrm{Cr}_{2} \mathrm{O}_{7}^{-2}+3 \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O} \rightarrow 3 \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O}_{2}+2 \mathrm{Cr}^{3+}+4 \mathrm{H}_{2} \mathrm{C} \) \( \quad 0.0975 \mathrm{M} \) \( 0.2925 \mathrm{M} \) \( 0.0325 \mathrm{M} \) \( 30.77 \mathrm{M} \)