Home /
Expert Answers /
Physics /
two-mol-of-an-ideal-gas-is-taken-through-a-reversible-cycle-as-shown-in-figure-b6-process-bc-i-pa860
(Solved): Two mol of an ideal gas is taken through a reversible cycle as shown in Figure B6. Process bc i ...
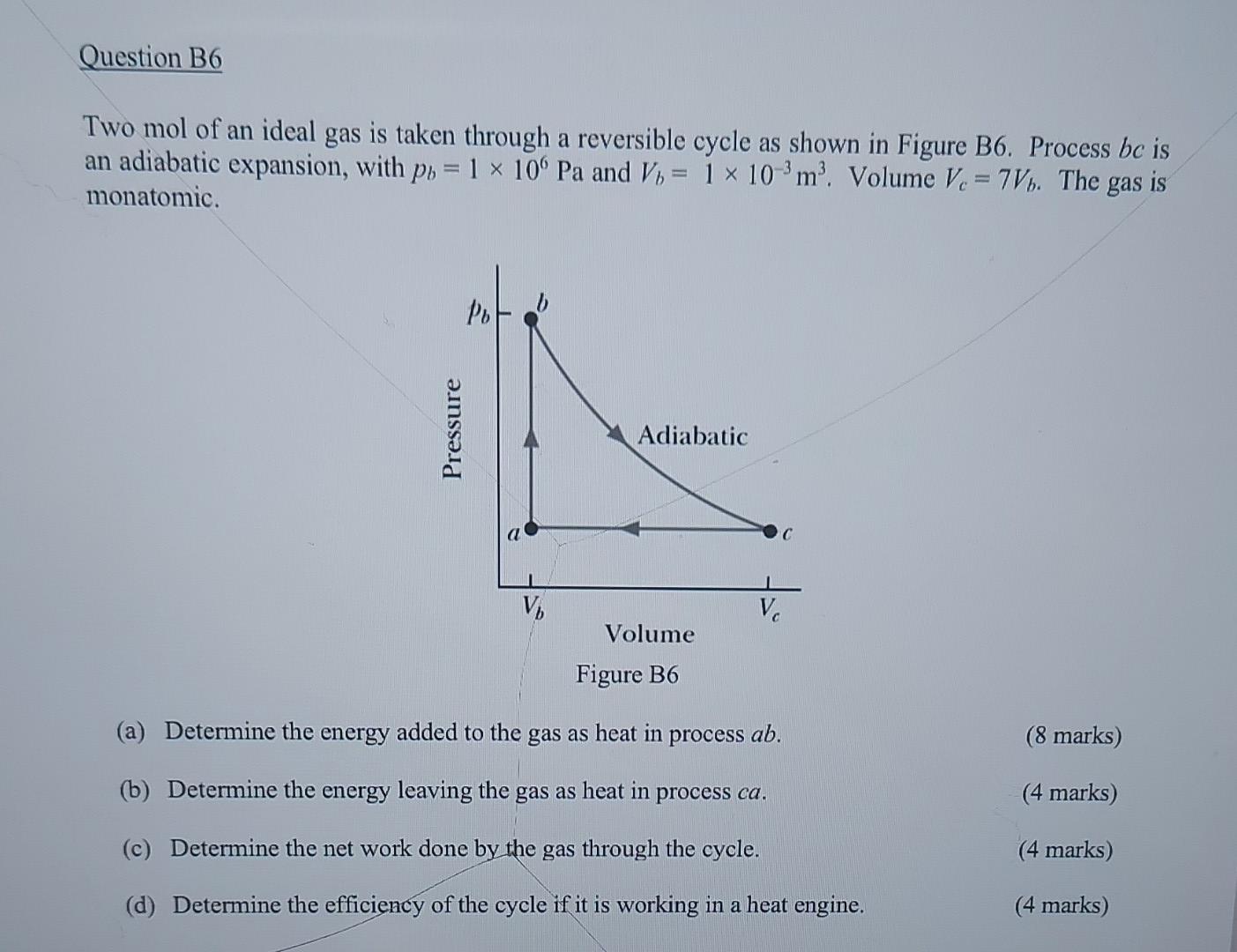
Two mol of an ideal gas is taken through a reversible cycle as shown in Figure B6. Process is an adiabatic expansion, with and . Volume . The gas is monatomic. (a) Determine the energy added to the gas as heat in process . (8 marks) (b) Determine the energy leaving the gas as heat in process . (4 marks) (c) Determine the net work done by the gas through the cycle. (4 marks) (d) Determine the efficiency of the cycle if it is working in a heat engine. (4 marks)