Home /
Expert Answers /
Chemistry /
two-experiments-were-conducted-in-a-bomb-calorimeter-the-first-experiment-was-to-determine-the-hea-pa991
(Solved): Two experiments were conducted in a bomb calorimeter. The first experiment was to determine the hea ...
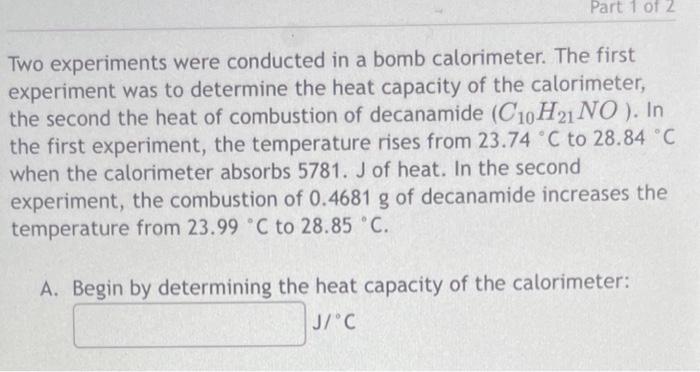
Two experiments were conducted in a bomb calorimeter. The first experiment was to determine the heat capacity of the calorimeter, the second the heat of combustion of decanamide . In the first experiment, the temperature rises from to when the calorimeter absorbs 5781. J of heat. In the second experiment, the combustion of of decanamide increases the temperature from to . A. Begin by determining the heat capacity of the calorimeter:
Expert Answer
Explanation:Explanation: To determine the heat capacity of the calorimeter, we can use th