Home /
Expert Answers /
Chemistry /
titration-of-sodium-acetate-a-10-00-ml-solution-of-0-1700-m-ch3coona-is-titrated-with-0-1300-m-hci-pa951
(Solved): Titration of Sodium Acetate A 10.00 mL solution of 0.1700 M CH3COONa is titrated with 0.1300 M HCI ...
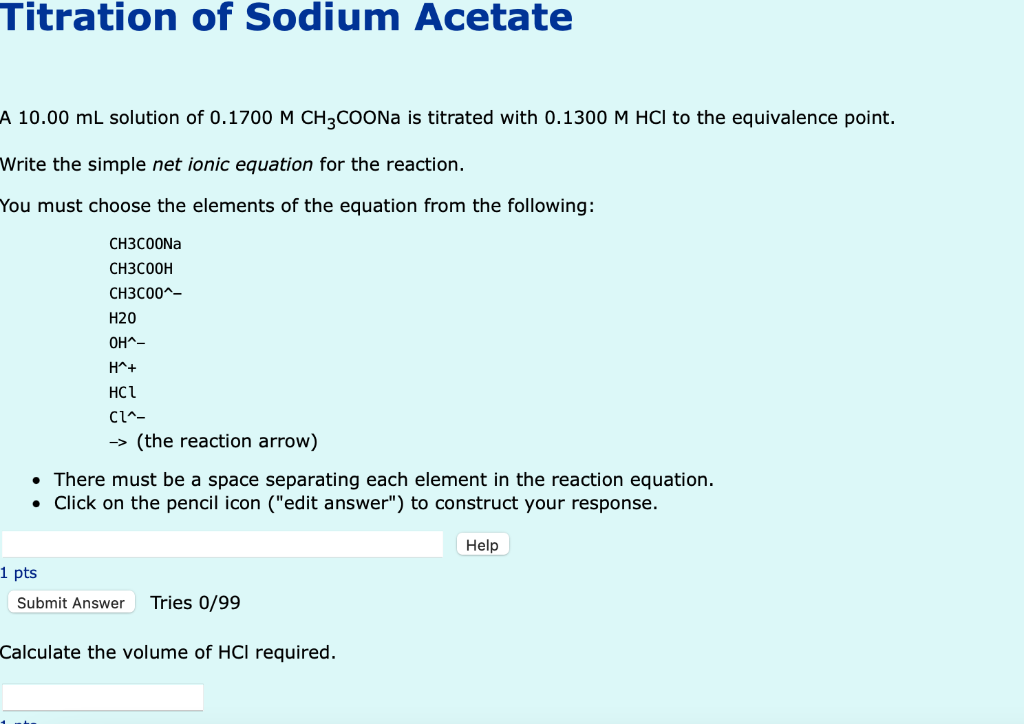
Titration of Sodium Acetate A 10.00 mL solution of 0.1700 M CH3COONa is titrated with 0.1300 M HCI to the equivalence point. Write the simple net ionic equation for the reaction. You must choose the elements of the equation from the following: CH3COONa CH3COOH CH3C00^- H20 OH^- H^+ HC1 CL^- -> (the reaction arrow) • There must be a space separating each element in the reaction equation. ? Click on the pencil icon ("edit answer") to construct your response. Help 1 pts Submit Answer Tries 0/99 Calculate the volume of HCI required.